Solar Cells Made From Quantum Dots
Next generation photovoltaic cells will use furturistic colloidal quantum dot technology.
Client statement:
"Our product is a solar cell made from quantum dots and absorbing light in the IR. That cell is stacked behind a regular silicon cell to act as a hybrid module. We want to run tests with your silicon wafer to simulate a thin silicon Photo Voltaic (PV) module."
The following thin silicon item was used for the research above.
Item# 3235 Thin Silicon 100mm P /B <100> 10-20 100um DSP
Quantum Dots are an Efficient and Versatile Solar Material
- Nanometer sized particles in solution
- Light absorption much more efficient than c-Si
- Absorption onset can be tuned into the infrared, where very few materials absorb well
Get Your Quote FAST! Or, Buy Online and Start Researching Today!
What is a Quantum Dot Solar Cell?
A Quantum Dot Solar Cell is a new type of photovoltaic cell that uses quantum dots as a primary absorbing material. It aims to replace bulk materials that are used in conventional solar cells. These tiny dots contain an enormous amount of energy that can be converted into electricity.
Quantum dots have shown great promise in solar cells. They were developed by the Los Alamos National Laboratory and have the advantage of being non-toxic and maintaining high efficiency. They are made of tiny circular crystals only a few nanometers across. Their color changes with slight variations in their size, making them extremely efficient at absorbing light. They have been used in many applications including TVs and sensors, as well as in solar cells.
This new technology works by using quantum dots to capture the excess energy from light that would normally be lost through heat generation. They work by reversing the way electrons and holes move inside a solar cell. As a result, a quantum dot solar cell can increase the efficiency of sunlight-to-electricity by 25 percent.
Quantum dots are also capable of tuning their absorption bandgap to match the wavelength of incident light. This property allows them to extract carriers without losing voltage. Because of this, quantum dot solar cells may have the potential to be more efficient light harvesters and energy converters.

What Use Quantum Dots in Solar Cells?
This is the perfect ideal for solar cells, because the band gap of the quantum dot can be adjusted. This will give us the opportunity to exploit the heterojunction and sensitize the carbon nanotubes of the solar cells with quantum dots. We have been granted a patent for the development of a new type of quantum point sensitized solar panel with the aim of promoting the production of inexpensive, high efficiency solar modules. Q DSCs and other quantum dot-sensitive solar cells will also be sensitized with graphene, a material with high thermal conductivity. [Sources: 1, 5, 11]
A quantum dot solar cell (QDSC) is a type of solar cell that uses quantum dots as a captivating and absorbing photovoltaic material. A quantum dot solar cell, or Q DSC, is the first of its kind in the world and is part of a new generation of solar cells that use quantum to create a fascinating photovoltaic material, according to researchers at the University of California, Berkeley. [Sources: 0, 1]
Quantum dot solar cells are a variant of this approach and use quantum mechanical effects to gain further power. A quantum dot - a sensitized solar cell or Q DSC - is similar to a dye - sensitizes solar cells by absorbing photovoltaics at a much higher rate than a conventional solar cell. [Sources: 5, 8]
For solar energy to be successful, we need those that can offer higher efficiencies and lower the cost of standard silicon solar cells. The efficiency of quantum dot solar cells (Q DSCs) and other quantum dots is relatively low compared to the silicon-based photovoltaic systems used today. [Sources: 6, 7]
The size dependence of the band gap allows to increase the efficiency of multiple solar cells by using materials that absorb all wavelengths of light that are found in the solar spectrum. The quantum dot solar cell is inexpensive to grow and offers the potential to produce highly efficient, cost-effective solar modules. QMC hopes to reduce the cost of quantum dots and other quantum dots - solar panels - by applying its nanomaterials to other emerging industries. This makes them an ideal candidate for use in a wide range of applications such as solar photovoltaics, wind turbines and solar power storage. [Sources: 7, 10, 12]
There is still a long way to go before the quantum dot solar cell can be commercialised, but the potential is great. There is still a lot of work to be done before it will be presented on a commercial basis. However, the potential for this is greater due to the high material costs and the need for high quality control. [Sources: 0, 6]
The technology for this solar cell is advancing rapidly and solar cells with quantum dots are seen as an encouraging solution for the future. There are many other potential applications we are talking about, such as solar energy storage, because quantum dot solar cells will offer a new way to use solar energy at a much lower cost than conventional solar cells. [Sources: 0, 6]
This is the quantum dot solar cell proposed by Barnham and Duggan in the 1990s and one of the first commercial applications of quantum dots for storing solar energy. The cost per square metre of solar cells is relatively low compared to silicon-based photovoltaic systems used today. [Sources: 7, 10]
To produce a quantum dot solar cell that can be sprayed or painted, tiny nanometer-sized semiconductors must be dispersed in a dispersed substance, a so-called colloid. In this solar cell design, quantum dots are used as a material to absorb sunlight and store energy. [Sources: 7, 9]
The band gap determines whether the region of the solar spectrum containing ultraviolet, visible and infrared light can be absorbed by the quantum dot solar cell and converted into electricity. The size dependence on the band gaps allows to increase the efficiency of multi-junction solar cells by using materials that absorb all wavelengths of light found in the solar spectrum. [Sources: 4, 7]
QDSCs are often used as sensitizers in campers, heat dissipation is better controlled and dye-sensitive solar cells could benefit from lower production costs than conventional solar panels and solar modules. Next time someone needs an additional PV panel, they might think about introducing a spray can to make their can. Quantum Dot solar cells, many of which are employed by companies, cost much less than conventional PV panels, such as the ones shown above. [Sources: 9, 11, 13]
One approach to solar cells uses quantum dots of only ten atoms to absorb sunlight and convert it into electricity. Tunable band gaps in the quantum dot allow nanostructured solar cells to harvest more of the solar spectrum. There are also plans to use layers of quantum dots of different sizes to make multi-junction solar cells that could absorb even more sunscreen. Many researchers who deal with quantum - full stop - sensitized solar cells have pursued other growth strategies to collect quantum on the electrode surface. [Sources: 1, 2, 3, 5]
One such approach, currently under investigation, is the production of quantum dot solar cells with a CDTE nanocrystals ratio of 1: 1,000 to 1.5. Tachan still needs further research to develop the ratio and CDte nanocrystals for quantum dot - sensitized solar cell and to design the quantum dots for their solid-state infrared radiation - visible upward conversion. He is investigating the potential to produce a single - atom - per - square meter (DCTE) -sensitized solar cell and is investigating its possible use in a wide range of photovoltaic applications. [Sources: 10, 11]
Sources:
[0]: https://www.altenergymag.com/article/2018/05/quantum-dot-solar-cells-are-coming/28547
[1]: https://solarfeeds.com/wiki/quantum-dot-solar-cell/
[2]: https://www.energy.gov/science/bes/articles/one-small-change-makes-solar-cells-more-efficient
[3]: https://www.solarnovus.com/quantum-dot-solar-cell-achievs-world-record-7-efficiency_N5475.html
[4]: https://www.offgridenergyindependence.com/articles/7518/promise-for-higher-efficiency-quantum-dot-solar-cells
[5]: https://www.intechopen.com/books/solar-cells-new-approaches-and-reviews/quantum-dots-solar-cells
[6]: https://medium.com/@alishbai734/artificial-atoms-to-harness-solar-power-a10f0f1a288c
[7]: https://genesisnanotech.wordpress.com/2017/04/22/doe-one-small-change-makes-quantum-dot-solar-cells-more-efficient/
[8]: https://wikimili.com/en/Quantum_dot_solar_cell
[9]: https://cleantechnica.com/2011/09/28/improvements-on-quantum-dot-solar-cells-show-remarkable-in-balancing-performance-with-cost/
[10]: http://171.67.100.116/courses/2014/ph240/singh1/
[11]: http://www.virtek.nl/wordpress/quantum-dot-sensitized-solar-cells/
[12]: https://en.wikipedia.org/wiki/Quantum_dot_solar_cell
[13]: https://solarpowerinvestor.com/quantum-dot-solar-cells-efficiency-as-the-future-of-solar/
Indium Phosphide Zinc Sulfide MPA COOH Quantum Dots
There is a common misconception that trapping is a necessary step for producing quantum dots. In fact, trapping is a crucial step for producing high-quality quantum dots, as it prevents the emission of photons at a high energy. Here's what you need to know about trapping within InP/ZnS quantum dots.
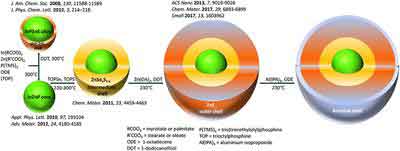
Synthesis
The synthesis of InP/ZnS quantum dots has been developed by Najing Tech Company. In this process, InP/ZnS quantum dots have been modified by adding carboxyl groups, hydroxyl groups, and amino groups. In this way, the resulting quantum dots have been characterized by TEM. Their morphology and size are described in Figure 1. The average diameter of aQDs is smaller than that of the other two types of InP/ZnS quantum dots.
The InP/ZnS quantum dots are a less toxic alternative to the cadmium-based QDs. Their size, shape, spectra, and hydrodynamics in aqueous solution were measured. The QDs were characterized by confocal laser scanning microscopy and flow cytometry, which was used to determine their uptake efficiency and qualitatively assess apoptosis and ROS production.
The luminous efficacy of InP quantum dots is affected by the quality of the quantum dot shell. InP quantum dots with poorly formed shells exhibit poor luminous properties, because the surface molecules affect their luminescence. In addition, InP quantum dots may be sensitive to ozone, which is harmful to the environment. Despite these advantages, there are still some problems with InP quantum dots.
The synthetic method developed in this study is suitable for mass production. However, the problem with InP quantum dots is that it is difficult to make them uniform in size, and it does not give very high yields compared to CdSe. The synthesis method also has limitations when it comes to growth of quantum dots, as they cannot grow beyond a certain size.
In this study, a bovine serum albumin was used as the matrix for synthesized AgInS2 nanoparticles. TEM analysis indicated that the nanoparticles had a stable dispersion and were 15.9 A+-2.1 nm in diameter. To determine the stoichiometric balance, the feed thiol-In(III) ratio was controlled. The pH level affected the protonation state of thiols and, hence, the formation of metal hydroxides.
QDs containing InP/ZnS-OH have a higher uptake efficiency compared to those with carboxyl or amino ends. Fluorescence imaging has shown similar results for both QDs at 20 mg/mL. They were tested on HCC-15 and RLE-6TN cells. They showed concentration dependent uptake of the two quantum dots.
Characterization
The chemistry and fate of Indium phosphide (InP) QDs were studied for several chemically distinct preparations. This study evaluated seven core-shell and indium-zinc-phosphide (InZnP) QDs. The surface and phosphorus moieties of the InZnP quantum dots were functionalized by penicillamine and glutathione.
InP/ZnS QDs exhibited low cytotoxicity in pristine form, while CdSe/ZnS-based quantum dots had high cytotoxicity. No surface functionalization significantly affected cytotoxicity and stability. No cytotoxicity was observed in cells exposed to InP/ZnS-based QDs, even after the formation of their oxidation products. Moreover, the In-Zn S gradient shells displayed a reduced toxicity compared with In-P-QDs. In-shell QDs were found to be stable in solution, whereas core-shell QDs exhibited high toxicity.
The InP/ZnS QDs exhibited a monodisperse size distribution with an average of five +/-0.5 nm. They contained carboxyl, hydroxyl, and amino surface groups, and their FT-IR spectra were similar to those of InP/ZnS QDs. The indium-zinc-phosphorus alloys showed the highest photosensitivity.
During the characterisation of InP/ZnS QDs in toluene solution, we used the FS5 Spectrofluometer with the SC-05 Cuvette Holder Module. For the time-correlated single photon counting, we used an EPL-405 pulsed diode laser. The FS5 Spectrofluorometer was used to acquire spectra.
The intrinsic toxicity of InP/ZnS QDs is much lower than those of heavy metals. InP/ZnS quantum dots exhibit a narrow color tunable luminescence. InP/ZnS quantum dots also have lower toxicities than their cadmium-based counterparts. Ultimately, InP/ZnS quantum dots are safer and more environmentally friendly than the latter.
Further studies are required to optimize the composition of Indium Phosphide and Zinc Sulphide quantum dots to minimize toxic intermediates. Nevertheless, it is clear that the research on Indium Phosphide (InPZS) MPA quantum dots has the potential to change the future of the field of quantum dots. The new research will improve our understanding of the chemical and biological properties of quantum dots.
In the past, this kind of research has yielded a lot of promising results. It has shown that these quantum dots can enhance DNA synthesis in a wide range of cells. Moreover, a study on InP/ZnS QDs in human breast cancer cells revealed that the MNPs are efficiently absorbed by the cell. Using multiple staining strategies, the researchers were able to monitor cell morphology and observe induced apoptosis and necrosis.
Applications
Indium Phosphide/Zinc Sulphide MPA COOH quantum dot semiconductors have unique photoluminescence properties that make them an excellent choice for applications in light-emitting diodes (LEDs). These nanostructured molecules can be produced using simple chemical reactions and are a promising alternative to conventional silicon solar cells. These quantum dots can also be manufactured inexpensively.
In vivo toxicity testing with QDs was conducted using mice. In vivo toxicity testing revealed that InZnPS QDs have a lower cytotoxic potential than core-only QDs. Moreover, a variety of surface functionalizations did not affect cytotoxicity. In addition, exposure to InZnPS QDs did not result in an excess of reactive oxygen species or oxidative damage to cellular DNA. In addition, accelerated weathering induced precipitation, degradation, and complexation of In and Zn ions with phosphate and carboxylate moies.
To study InP/ZnS QDs, a series of experiments were conducted. First, InP/ZnS QDs were prepared by dilution with toluene and precipitated in ethanol, followed by resuspended in toluene. Second, indium was measured using ICP-MS (7500C1 Agilent, United States) by determining the amount of indium within the samples.
The InP/ZnS QDs were injected into mice with different surface modifications. hQDs contained a COOH moiety, whereas aQDs contained a NH2 moiety. After the injection, the InP/ZnS QDs were assessed for hematology, cytotoxicity, and blood biochemistry.
QDs-applied in biosensors are promising because of their biocompatibility and high photodegradation resistance. In addition, QDs-oleylamine were reported to have average size ranges of 2-5 nm. Further, indium-based QDs-oleylamine (Oly) were synthesized in a previous study.
The nanoscale nature of Indium Phosphide (InP) and Zinc Sulphide MPA COOH crystalline semiconductors has facilitated the discovery of many applications for these materials. While the biodistribution and toxicity of InP/ZnS QDs remain unknown, these quantum dots show promising potential for biomedical applications.
The toxicity of InP/ZnS quantum dots is largely unknown, as few studies have been performed on InP/ZnS QDs. However, InP/ZnS nanocrystals exhibit higher levels of covalent bonding, making them safer than cadmium-based QDs. Chibli et al. found small amounts of hydroxyl radicals in the cytoplasm of HCC-15 cells.
Trapping within Indium Phosphide Zinc Sulphide MPA COOH quantum dots
The potential for biomedical applications of Indium Phosphide Zinc Sulfide MPA COOH quantum dots is broad. However, they are largely unknown in terms of toxicity and biodistribution, and this has hindered their development. The following paragraphs will discuss some of the research that has been done with InP/ZnS quantum dots.
Mn/ZnS/ZnS quantum dots are synthesized by a one-pot synthesis using a stabilizer, 3-mercaptopropionic acid. Mn-ZnS/ZnS quantum dots have a diameter of about 2.9 nm and a shell diameter of 1.9 nm. UV-Vis spectroscopy and fluorescence spectroscopy have been used to investigate the optical properties of Mn/ZnS quantum dots.
The toxicity profile of InP/ZnS QDs was assessed using mouse experiments. Animals were exposed to a high-dose of InP/ZnS QDs for 3 days. Acute inflammatory reaction and slight changes in liver function were reported. These results suggest that different surface modifications are crucial for the in vivo toxicity of InP/ZnS QDs, and must be taken into account when synthesizing QDs in the future. Clinical transformation of QDs will be a long process, but with rapid progress in technology, the timetable to a full biological application is likely to be shorter than expected.
A large number of materials have been investigated for their potential for the application of quantum dots. Indium Phosphide Zinc Sulphide MPA COOH quantum dots have been studied extensively. The results obtained with these materials indicate that the core/shell structure is the most stable among the three. However, the thickness of the core shell and the core-shell structure influence the diamagnetic susceptibility of the donor.
In addition to the zinc sulfide MPA COOH quantum dots, zinc sulfide nanowire heterojunctions have been prepared from thermal evaporated powders. These nanowires exhibit a high degree of stability and are well-dispersed. Electron microscopy, X-ray powder diffraction, and high-resolution transmission electron microscope have all been used to study the Zn/Cd interface structure.
