CdTe Costs Less than Silicon Solar
Not only is the cost of traditional silicon photovoltaics high, but it also takes up a lot of space. This makes it difficult to install solar panels in certain areas or on certain buildings.
 Cadmium Telluride based solar cells are more efficient than traditional silicon photovoltaics and other thin-film solar technologies. They are also less expensive to produce, which means that the cost of installing them will be lower.
Cadmium Telluride based solar cells are more efficient than traditional silicon photovoltaics and other thin-film solar technologies. They are also less expensive to produce, which means that the cost of installing them will be lower.
Get Your Quote FAST! Buy Online and Start Your Research Today!
Cadmium Telluride (CdTe) Substrates
Below are just some of the CdTe wafers that we have in stock:
- CdTe (100), undoped, P-type 5x5x1.0 mm, SSP
- CdTe (100), undoped, P-type 5x5x1.0 mm, DSP
- CdTe (110), undoped, P-type 5x5x1.0 mm, SSP
- CdTe (110), undoped, P-type 5x5x1.0 mm, DSP
- CdTe (111) A, Cd-terminated, undoped, P-type 5x5x0.4-0.5 mm, SSP
- CdTe (111) B, undoped, P-type 5x5x0.4 mm, SSP
- CdTe, Undoped, P-type, (110) 10x10x1.0 mm, DSP
- CdTe (100) , undoped, P-type 10x10x1.0 mm, SSP
- CdTe doped with Zn, P type , (CZT ) (111) 10x10x 1.0mm, DSP
- CdZnTe (111) P type, 5x5x0.5mm SSP
- CdZnTe (111)B, with Zn concentration around 14% , P type, 10.5x10.5x1.0mm 1sp R:>1E6 ohm.cm
- CdTe (111), Undoped, P-type 10x10x0.5 mm, SSP
- CdTe (111), Undoped, P-type 10x10x0.9 mm, DSP
- CdTe (111), Undoped, P-type 15x15x0.5 mm, SSP
- CdTe (111)B, Undoped, P-type 10x10x1.0 mm, SSP with B face to be polished
- Hg(1-x)Cd(x)Te, x=0.17, Undoped, N-type 14x12x0.5 mm, DSP
What Is Cadmium Telluride Substrates Used For Solar Cells?
Cadmium Telluride
Cadmium Telluride (CdTe) is a semiconductor material that is useful for making solar cells. Unlike traditional 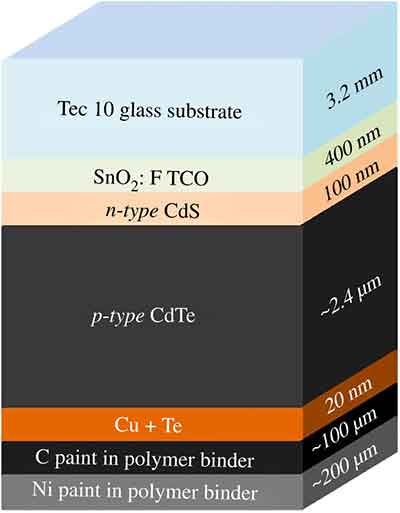 silicon solar cells, CdTe solar cells are very efficient and less expensive to produce and install. The following are some advantages of CdTe solar cells.
silicon solar cells, CdTe solar cells are very efficient and less expensive to produce and install. The following are some advantages of CdTe solar cells.
The material can be formed into multiple layers, with each layer having a different alloy composition. The composition of each layer is determined by the partial pressure. The temperature of the substrate is also crucial. The substrate can be annealed at up to 200deg C. for a short period of time.
The material can be used for spectroscopy and solar cells. It also has a deep IR transmission range. It is available in crystal or powder form. The transmission graphs can be enlarged by clicking on the images. The data is also available in pdf format. If you'd like to buy sheets of Cadmium Telluride, you can view more details here:
The disadvantages of using this material include the fact that it contains cadmium, which is a toxic metal. The acute effects can include respiratory edema and pulmonary damage. Cadmium is insoluble in water, so it may not be as toxic as cdTe, although further research is necessary.
The growth of cadmium telluride films on cadmium telluride substrates has been investigated using chemical vapour deposition. A transporting agent was hydrogen. Several deposition runs were carried out to determine the most effective growth conditions. The resulting films were characterized by optical and scanning electron microscopy, and Auger electron spectroscopy. In addition to this, X-ray diffraction and Auger electron spectroscopy were used to study the films' physical properties. The best films had good epitaxial relationship. The films also contained pinholes and hollow crystalline grains.
Cadmium Telluride (CdTe) semiconductors are a popular material in a variety of applications. It is a direct-bandgap semiconductor with a bandgap of about 1.5 eV at room temperature, which is optimal for converting sunlight into electricity. The material can be used for solar cells, lasers, photoresistors, and ionizing radiation detectors.
Its photovoltaic properties
Cadmium Telluride is a relatively rare element with photovoltaic properties that are similar to those of silicon. However, the efficiency of CdTe solar cells is less than that of silicon. One reason for this is the scarcity of tellurium, which is extremely rare. It is found only in a few parts per billion in the Earth's crust. In 2007, there were only 135 metric tons of tellurium produced globally. This metal is mostly obtained as a byproduct of copper refining, and in lesser quantities from gold and lead. This means that CdTe PV modules will be limited in their efficiency.
Research on CdTe has been conducted since the 1950s. The material absorbs energy from the sun at shorter wavelengths, which makes it easier to convert to electricity. This makes the material cheaper to produce and install compared to conventional solar panels. It is also environmentally friendly compared to silicon, making it a good choice for use in solar panels.
While the physics behind CdTe are still in the early stages, the materials are starting to catch on and become more accessible to consumers. The first commercially available CdTe solar cell is a low-cost multi-kilowatt system. Moreover, it is one of the most widely used thin film PVs. However, the downside of CdTe is that it is not as cheap as silicon-based panels.
The environmental benefits of CdTe solar cells outweigh the disadvantages. The technology is environmentally friendly, and the use of CdTe solar cells reduces cadmium emissions in the atmosphere. However, there are some health risks associated with the production of CdTe solar cells. The chemicals used in the manufacturing of CdTe solar cells are liquid or powder, and can disperse, thereby exposing workers to the cadmium compounds through air or hand-to-mouth contact.
Its application in solar cells
Solar cells made of cadmium telluride are more environmentally friendly than silicon cells, and they are cheaper to produce. They also absorb more of the sun's energy and can convert more of it into electricity. This makes them more cost-effective and more efficient to produce, as they are easier to install.
While silicon dominates the solar cell market, CdTe is a growing contender, and laboratory-scale devices are reaching efficiencies of over 18%. This paper discusses the fabrication process for CdTe-based polycrystalline thin-film solar cells, including common substrates, constituent layers, and interfaces. We also discuss common tricks used to increase CdTe efficiency.
A CdTe-based solar cell is composed of two different thin film layers, a window layer and a p-type CdTe absorber. The windows and absorbers are connected by a conductive rear surface, and light passes through them.
The cadmium telluride layer is layered on a glass substrate. The surface of the superstrate is made of a glass substrate coated with sodium. In this process, sodium is deposited on the glass substrate, which improves the cell efficiency.
A CdTe-based solar cell starts with a clean glass substrate. Then, the transparent conducting oxide layer (TCO) layer is placed on top, which is known as the TCO. This layer allows light to reach the semiconductor and provides the top contact needed to extract power from the cell. After this, the p-n junction is formed.
The manufacturing process of CdTe solar cells presents significant hazards for workers and the environment. Inhalation of cadmium telluride is a significant health risk. The chemical is highly toxic and can cause pulmonary edema in humans.
Its toxicity
Cadmium Telluride (CdTe) is an emerging technology that can be used to produce solar cells. It is considered a potentially hazardous substance because of its toxicity. Exposure to CdTe can cause pulmonary edema and pneumonia. Some studies have suggested that cadmium may be less toxic than cdTe, but more research is needed to determine this.
There are several disadvantages to Cadmium Telluride, including its toxicity and a limited global supply. The unencapsulated cells are highly sensitive to moisture, and this toxicity limits market growth. The toxicity issue also limits public acceptance. However, relatively small-scale production has begun in the United States, Japan, and Germany. However, the major players ceased production in Germany in 2002.
Although this material is useful for manufacturing quantum dots, it has been controversial for its toxicity. Toxicological studies of quantum dots are necessary before their use in humans. Only a few in vivo studies have been conducted to date, and their toxicology remains controversial. Therefore, the current study aimed to understand QD toxicity across different time points, including the effects of free cadmium ions and hydroxyl radicals on tissue damage. It also examined kidney morphology and liver function.
Cadmium Telluride is also used to produce highly efficient thin film solar cells. These cells use a n-i-p structure and achieve efficiency levels as high as 10%, which is lower than silicon solar cells. However, because of Tellurium's high toxicity and low supply, it is limited in use.
Its production
The process of forming polycrystalline films from cadmium telluride requires a substrate. In this process, a substrate is placed in an inert gas atmosphere and heated to a temperature sufficient to produce the polycrystalline film. Once the substrate reaches the annealing temperature, the cadmium telluride is deposited on the substrate.
QUESANT QScope-250 atomic force microscope was used to study the formation of cadmium chalcogenide on PA 6. The atomic force microscope was used in contact mode to examine dry samples. The cantilevers CSG10 series were used with a force constant of 0.2 Nm-1 and a nm tip curvature. SPIP software was used to analyze the images. The results showed that a cadmium chalcogenide layer adheres strongly to the polymer substrate.
Cadmium Telluride is a stable crystalline compound composed of cadmium and tellurium. Its low-density structure provides excellent photovoltaic properties, as well as a high optical absorption coefficient. The compound's narrow bandgap allows it to convert sunlight into electricity with a theoretical efficiency of about 32%.
Cadmium Telluride, also known as CdTe, is used in the production of solar cells. The material is used to produce photovoltaic cells and laser windows. It is also used in photothermal conversion. The production of solar cells requires a variety of common semiconductor materials. The chemical properties of these semiconductors determine the efficiency of the solar cells.
This material has potential to be widely used in photovoltaic energy generation. However, because of its potential for extensive human interfaces, definitive toxicological studies are needed to ensure that the process is safe for human and aquatic life.
What are the Advantages and Disadvantages of Cadmium Telluride Solar Cells?
The advantages of using cadmium telluride solar cells are many. First of all, they have a much lower cost of  manufacturing. Because they absorb the sun's energy at shorter wavelengths, they produce a higher energy density which is easier to convert to electricity. Second of all, they are less expensive to install than silicon panels. Third of all, they are more environmentally friendly. And finally, they are also more cost effective to produce.
manufacturing. Because they absorb the sun's energy at shorter wavelengths, they produce a higher energy density which is easier to convert to electricity. Second of all, they are less expensive to install than silicon panels. Third of all, they are more environmentally friendly. And finally, they are also more cost effective to produce.
There are a number of concerns about the environment when using cadmium telluride solar cells. One of the most pressing concerns is the environmental impact of this material. However, there is no need to worry, because telluride is abundant in all of Earth's seafloor. Therefore, the shortage of telluride will not be a big problem in the long run. But there are some drawbacks.
The main disadvantage of cadmium telluride solar cells is that they have relatively high production costs. But this is offset by the increased efficiency. The high-efficiency of cadmium telluride solar cell technology has made it more expensive than its silicon counterpart. Moreover, the technology is easier to scale. Once the technology is proven, manufacturers can manufacture complete modules in a matter of hours. That means that a large number of consumers will benefit from this
CdTe Solar Efficiency
The methods described here have lower costs than those used to produce cadmium telluride films. The reported efficiency of the CdTe - Beat Silicon - CIGS Technology Cad mite substrates are obtained with CSS deposition methods, with the highest efficiency for the substrate (polyIMIDE) being 7.3%, while the lowest efficiency for a thin film (non-cd Te) substrate is 7-8% (25-28%).
CdTe Solar Cells
CdTe based solar cells is lowering the cost of solar panels. The material is more efficient than traditional silicon photovoltaics and other thin-film solar technologies. Currently, the price of the raw Cad mite material , or "tellurium," is 10-20% lower than that of silicon, and at the same time the production costs for the production of Cadmium - telluid foils - are also lower, which reduces the cost of capital and at the same time the total cost per kilowatt hour - the hour of a CdTe - on a photivoltaic basis, according to the researchers.
Cadmium Telluride Solar Cells Research
A new deposition method created at the University of California, San Diego (UCSD) and the National Renewable Energy Laboratory (NREL) to fabricate highly efficient CdTe solar cells using a CSS deposition method. This requires the use of thin polyimides deposited onto a superstrate structur.
