Can you fabricate a custom stack involving ITO, amorphous hydrogenated silicon and SiO2? I do not see a-Si-h listed on your website. We just need some simple, maybe ideally straight, waveguides for some tests. The details shouldn't matter too much. For the amorphous silicon custom device, yes we would have precise requirements on thicknesses."
Amorphous Silicon Used in Research
A scientist asked the following:
Please reference #250469 for specs/pricing.
Get Your Quote FAST! Or, Buy Online and Start Researching Today!
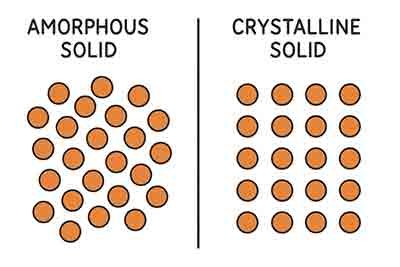
5 Differences Differences Between Amorphous & Crystalline Solids
Below are 5 differences to consider when choosing to research amorphous & crystalline solids.
Crystalline Solids
- Crystalline Solid has an orderly arrangement of their constituent particle.
- Crystal has a SPECIFIC GEOMETRY SHAPE WITH DEFINITE EDGE
- Crystalline solid cleavage along particular point & direction.
- Crystalline solids have a sharp melting point
- They are also known as true solids.
Amorphous Solid
- Amorphous solid has no such arrangement. Their practices are randomly organised.
- They do not have geometry in their share.
- Amorphous solid cleavage into uneven parts with ragged edges.
- They will have a range of temperature ovens which will melt.
- They are also known as supercooled liquid.
Characteristics
2.) The following characteristics define a unit cell.
- A unit cell has three edges a,b & c and three angles ( ?),B & V between the respective edges
- the a,b & c may not be mutually perpendicular
- the angle between edge b & c is ( ?), and a & c is B and that of between a & b is v.
Determining The Difference Between Crystalline & Amorphous Solids
The fundamental difference between crystalline and amorphous compounds is the arrangement of their constituent atoms. A crystalline solid has a long range of ordered molecules and a sharp melting point. In contrast, an amorphous compound has a short range of ordered molecules and an irregular arrangement of its atoms. This makes amorphous compounds highly rigid and have irregular surfaces.
Amorphous solids are non-crystalline substances that do not possess a characteristic geometrical arrangement. They also do not have a fixed melting point. Despite their name, amorphous materials do display an orderly arrangement of atoms that may extend up to a few Angstrom units. Such structures are called crystallites. Amorphous solids lack distinct edges and are therefore non-crystalline.
Amorphous solids lack a long-range arrangement of atoms. In addition, they do not have any characteristic geometry. They are identical in all their properties along all their axes. Because of this, they are impossible to identify by their structure as a crystalline one. The distinction between amorphous and crystalline solids is very useful when describing the properties of different materials.
The difference between crystalline and amorphous material is based on their melting points and their cleavages. Crystals have definite melting points and their constituents are arranged in an orderly fashion. Amorphous materials do not have definite melting points. As a result, they are unstable. This means they can be easily broken and are often not re-usable for industrial processes.
The difference between crystalline and amorphous materials is primarily determined by the degree of ordering. Amorphous materials have no sharp melting point and lack definite structure. The amorphous solids have a wide range of melting and have no characteristic shape. They are flammable. They do not have definite structures. They have no definite defining temperature.
Amorphous solids are characterized by irregular breakage. They do not have definite melting points. They are homogeneous, asymmetrical, and have no sharp edges. Amorphous materials do not have a defined shape. Unlike crystalline solids, amorphous solids do not show long-range order. They are anisotropic.
A crystalline solid is rigid and has a regular geometry. It is characterized by a sharp melting point and a definite melting temperature. An amorphous solid has no definite or characteristic heat of fusion. It is amorphous. Amorphous solids are often more dense than crystalline ones. Amorphous solids are amorphous.
The difference between crystalline and amorphous is mainly based on the structure. The former has a sharp melting point and is brittle. Amorphous solids are softer and more pliable than crystalline ones. They are anisotropic. This means that they have no definite edges. They are polar and amorphous. But both are polar.
Amorphous solids are amorphous. Their structure is non-crystalline. This means that they do not have any sharp melting point and are non-crystalline. They are characterized by asymmetrical breakage. They are homogeneous and have no long-range order. There are many differences between the two types of solids. You can distinguish them by their physical properties by determining their melting and boiling points.
Amorphous solids have irregular surfaces and are amorphous. Amorphous solids have no defined shapes and cannot be cooled rapidly. In fact, rapid cooling of amorphous materials can cause them to become glass. This property can result in an amorphous material with poor-defined shapes and low density. If the cooling rate is too fast, the material will turn into a liquid.
Amorphous solids are amorphous in nature and lack a definite structure. In general, amorphous materials are made up of molecules that are irregularly arranged. Amorphous materials are similar to crystalline materials, but are not the same type. They are referred to as "amorphous" and are used for various purposes. The term amorphous refers to amorphous plastics.
Amorphous & Crystalline Solids Lecture
What Does Amorphous Mean?
What does Amorphous mean? This term describes a solid that lacks definite form or pattern. It is characterized by the lack of pattern, structure, or organization. As a result, it is difficult to define and may even be a cause of infringement. In addition, amorphous materials do not have a sharp melting point and are usually isotropic. In addition, they have no crystalline structure and are generally amorphous.
In chemistry, amorphous materials are those that lack a crystal structure, meaning that their structure is irregular. The term "amorphous" comes from the Greek words elastin and tropos, which mean the same direction. Because amorphous materials do not possess a geometric shape, they do not have sharp melting points. These materials are widely used in many fields, including plastic, rubber, polymers, and other household materials.
Another common definition of amorphous materials is "lacks a crystalline form". Amorphous solids are defined as those that do not have any definite shape. These substances are typically hard or plastic, and do not show any definite crystalline structure. However, there are many other kinds of amorphous objects. Some of these substances, such as amorphous minerals, lack a crystalline structure.
Amorphous materials lack a consistent form. This makes them difficult to manipulate or identify. These materials can be metallic, which is not as common as crystalline materials. Amorphous metals are difficult to create or find. Therefore, they are not used much in manufacturing. They are difficult to use and difficult to find. This is because amorphous materials have a crystalline structure, and therefore have a very low strength.
Amorphous Materials
What are Amorphous materials? They are solids that lack long-range order. They were once used to describe glass. However, these solids have no crystal structure and are typically not crystalline. Here's a closer look at amorphous materials. Here are some examples. We'll discuss amorphous metals and glass. This article focuses on the differences between amorphous and crystalline metals and their applications.
Amorphous solids are the most common types of solids. They don't have any crystalline structure and have no ordered or periodic structure. Amorphous materials are similar to crystalline ones, but their average atomic structure is different. Many of the solids we use in our daily lives are amorphous. They are a common type of plastic, and their properties make them useful in a wide range of applications.
Amorphous materials are solids that do not have a crystalline structure. Instead, they are characterized by their non-crystalline state. Their atomic structures are complex, and they can only become ordered with a lot of difficulty. The most common method to make amorphous materials is to rapidly cool a liquid or molten material. This decreases the mobility of the material's molecules, resulting in an amorphous solid.
Amorphous materials are generally made of a single material, but some can be amorphous in nature. Amorphous carbon, for example, is one type of amorphous carbon. In its natural state, this type of material has a crystalline structure, but the formation of impurities and defects disrupts the stable lattice. Moreover, the process of manufacturing amorphous carbon can be a very time-consuming and expensive process.
Video: Amorphous Materials Explained
What is The Definition of Amorphous?
The term "amorphous" refers to something that lacks a defined shape or structure. It is used to describe a substance or material that does not have a regular, repeating pattern of atoms or molecules, and therefore lacks a distinct crystalline structure. Amorphous materials can be solids, liquids, or gases and can range from simple molecules like water to complex polymers like plastics. Unlike crystalline materials, which have a well-defined arrangement of atoms or molecules, amorphous materials have a more disordered or random arrangement. Examples of amorphous materials include glass, rubber, and some types of metals.
Examples of Amorphous Solids
Amorphous solids are characterized by a lack of long-range order in their atomic or molecular structures. Here are some examples:
-
Glass: One of the most common examples, including window glass, glass containers, and fiberglass.
-
Plastics: Various types of polymers used in everyday items.
-
Gels: Substances that exhibit properties between those of liquids and solids, like gelatin.
-
Rubber: Used in tires, footwear, and various flexible products.
-
Amorphous Metals (Metallic Glasses): Alloys cooled rapidly to prevent crystal formation, used in electrical applications and high-strength materials.
-
Thin Film Coatings: Used in optics and electronics, such as anti-reflective coatings.
-
Pitch: A viscous, tar-like substance.
-
Silicon Dioxide (Silica) in Certain Forms: Such as fused silica used in optics.
-
Some Types of Wax: Like paraffin wax.
-
Foams: Polyurethane foam used in mattresses and insulation.
These materials find widespread use in various industries due to their unique properties, like flexibility, moldability, and optical characteristics.
Thermal Oxide Grown on Amorphous Silicon Wafers
A postdoc requested a quote on the following:
" I am looking for amorphous silicon on grown on top of SiO2 on silicon substrate. Whatever thickness and diameter you can provide with shortest lead time please provide a quote. If we can do 200mm we would need 10 wafers. In terms of specs, we are not too concerned. Let’s say 3kA SiO2 on the (100) Si substrate. And for the amorphous silicon please let me know what you can do for thicknesses. Is 3kA possible? Would price depend on amorphous si thickness?"
Two more questions:
- Would 1kA SiO2 vs 3kA SiO2 impact the price? We are ok with 1kA SiO2.
- If we reduce lot size to 5 wafers will it impact price per wafer?
UniversityWafer, Inc. Replied
We can do amorphous Si 200nm to 300nm!
Reference # 271938 for specs and pricing.
What is the difference between crystalline and amorphous materials? How do each of these materials behave when subjected to heat or pressure?
Crystalline and amorphous materials are two different types of solids that have distinct structural and behavioral characteristics due to the arrangement of their constituent atoms, molecules, or ions.
Crystalline materials have a regular, repeating arrangement of their constituent particles, forming a well-defined, ordered lattice structure. The periodic arrangement of particles gives rise to distinct properties, such as sharp melting points, anisotropy, and the ability to diffract X-rays. Examples of crystalline materials include most metals, minerals, and some polymers.
Amorphous materials, on the other hand, lack a long-range ordered structure. The arrangement of their constituent particles is random and disordered, similar to that in liquids but with more rigidity. Amorphous materials typically have a wide temperature range over which they soften or transition from a rigid state to a more rubbery or viscous state, instead of a sharp melting point. Examples of amorphous materials include glasses, gels, and some polymers.
When subjected to heat:
-
Crystalline materials: They generally have a well-defined, sharp melting point, which is the temperature at which the material transitions from a solid to a liquid state. As the temperature increases, the thermal energy causes the constituent particles to vibrate more intensely, eventually breaking the bonds that hold them in the lattice structure.
-
Amorphous materials: They do not have a sharp melting point. Instead, they undergo a glass transition, a range of temperatures over which the material transitions from a rigid, glassy state to a more rubbery or viscous state. The lack of a long-range order in amorphous materials makes it difficult for them to experience a sudden phase change, as observed in crystalline materials.
When subjected to pressure:
-
Crystalline materials: Due to their well-ordered structure, crystalline materials can exhibit anisotropic mechanical properties, meaning their properties may vary depending on the direction of the applied pressure. They can undergo phase transitions or deformation under pressure, depending on the strength of the bonds within the lattice and the specific crystal structure.
-
Amorphous materials: Since they lack a long-range ordered structure, amorphous materials generally exhibit isotropic properties, meaning their properties are the same in all directions. They tend to deform more uniformly under pressure, but the response to pressure is highly dependent on the specific material and its atomic or molecular interactions.
In summary, the primary difference between crystalline and amorphous materials lies in the arrangement of their constituent particles. Crystalline materials have a regular, ordered lattice structure, while amorphous materials have a random, disordered arrangement. This difference in structure leads to distinct behaviors when subjected to heat or pressure. Crystalline materials have sharp melting points and can exhibit anisotropic properties, while amorphous materials undergo a glass transition and generally exhibit isotropic properties.
Amorphous And Crystalline Solids
Amorphous solids are a type of non-crystalline substance. They do not have a definite melting point and are characterized by irregular breakage. They are amorphous solids because they are homogeneous and have long-range order. They are asymmetrical, meaning that their physical properties depend on direction. However, they are similar in other ways, and are often confused with crystals.
Amorphous solids, on the other hand, do not exhibit long-range order. This means that their properties are random and cannot be distinguished by their structure. This is one of the main differences between crystalline and amorphous solids. As the name implies, amorphous solids have no definite shape or geometric structure. They tend to break up into pieces that are irregular, and uneven.
The first difference between amorphous and crystalline solids lies in the structure of their molecules. While amorphous solids are not symmetrical and lack any definite geometry, they do have an orderly arrangement of atoms. These irregular atoms are known as crystallites. These materials do not have sharp melting points, and they do not change shape with temperature.
What Are the Differences Between Amorphous & Crysts? This article will explain the differences between crystalline and amorphous solids. These materials have a pronounced anisotropy, meaning that their properties change in different directions. Amorphous solids tend to be more dense, but they are still crystalline solids. This is one of the most important differences between solids.
Amorphous vs Cyrstalline Solids |
||
| Amorphous Solid | Crystalline Solid | |
| Definition | Amorphous solid is a form of solid that lacks a crystalline structure | Crystalline Solid or crystals have ordered structures and symmetry |
| Repeating Unit | No repeating units | There is one repeating pattern, thus we can observe the repeating unit. |
| Melting Point | Have no sharp melting point | Have a sharp melting point |
| Chemical Nature | Anisotropic | Isotropic |
What is a Crystal Substance?
A crystal is a solid material in which the constituents are organized in a highly ordered microscopic structure. This structure forms a lattice, which extends in all directions. These elements are known as the atoms of a crystal, and their arrangement within it is the basis for its properties. When a crystalline substance is formed, it retains its original shape and consistency. It can be used as a guide when examining crystallization.
The chemical bonds and particle types of a crystalline substance are the basis of its physical properties. There are four basic kinds of crystals: ionic, metallic, covalent network, and molecular. Each of these is characterized by its melting point, chemical properties, and molecular structure. Each of these atomic groups have a distinct set of properties. Here are some examples of each type. This article will explore each type of crystalline substance and give you an understanding of the properties of each.
A crystalline substance is composed of identical atoms or molecules. They can be a single solid, or a composite composed of many microscopic crystals. A polycrystal is made up of many smaller crystals called grains or crystallites. An amorphous solid lacks a periodic arrangement. The crystalline state is a spherical form of a solid that is shaped like a cube.
The degree of crystallinity of a substance is also defined by its chemical bonding. A metallic crystal is a crystal composed of metal cations surrounded by valence electrons. A molecular crystal, on the other hand, is made of molecules that are covalently bonded to each other. A crystalline solid has three-dimensional structure, a repeating pattern, and a high melting and boiling point.
There are different types of crystalline solids. Some are ionic, while others have a layered structure. A crystalline substance is a solid made of atoms and molecules. Its structure is a three-dimensional pattern of atoms and molecules. Its melting point is high. Its structure is very stable and can conduct electricity as a liquid. Some of the crystalline substances have defects that are incompatible with their desired properties.
The structure of a crystalline substance depends on its chemical bonding. A crystalline solid has a pattern of atoms that repeats itself. It is also a solid, but it contains defects. Its ionic properties are a result of its ionic bonding. Its structure is very rigid and has a high mechanical strength. There are four main types of crystalline substances: water, ionic, metallic, and covalent network.
The most common type of crystalline substance is quartz, but other solids can also be amorphous. An amorphous material is amorphous and is not solid. It is made of molecules and atoms in a non-crystalline form. However, it is amorphous. Therefore, the name amorphous solid refers to it as amorphous. Its amorphous.
The differences between a crystalline and amorphous substances are quite simple. A crystalline solid is a solid with a high melting point. An amorphous one does not have a high melting point. It is a solid with a low melting point and is a liquid. A amorphous material is amorphous, and is amorphous. The term amorphous is the same as ionic.
A crystal is a solid that has an ordered arrangement of atoms and molecules. It is composed of a single unit cell. A crystalline substance is not a liquid. It can be either amorphous or crystalline. A crystalline substance can have one or more atoms. This structure allows for the formation of a solid. As with any material, a crystal can be amorphous.
Crystals have characteristic angles. Their faces intersect at a fixed angle, which gives them their characteristic angle. X-rays can identify these angles. If a crystal is a liquid, it will have a crystalline structure. If it is a solid, it is the same as a liquid. A crystalline substance has a definite temperature range. A crystalline material will have a higher melting point than a liquid.
How are Crystalline and Amorphous Solids Similar
While crystalline solids are very structured, amorphous ones are not. Their melting points differ. Amorphous solids have a sharp melting point and are amorphous. Amorphous solids do not have definite edges. They are amorphous. Amorphous is more flexible, and is more malleable. This is a distinctly different material from crystalline.
In addition to their different properties, solids are also classified according to their structure. Unlike liquids, crystalline solids are not liquids and are not solids. They differ based on their atomic composition. They are both characterized by their chemical and physical properties, but the main difference is in their structure. Amorphous solids are softer and more pliable than crystalline ones, and are not brittle.
Amorphous solids are more pliable than crystalline ones. They are more prone to break than crystalline ones. Amorphous solids are amorphous and non-crystalline. Amorphous solids have no definite structure, and they are amorphous. They are not amorphous, but they are still in a liquid state.
Amorphous solids are amorphous, but they are different from crystalline ones in many ways. They lack a defined melting point and long-range order. While crystalline solids have a definite melting point, amorphous solids are not. They are isotropic, meaning that they have no specific shape. Amorphous solids are highly flammable, whereas amorphous ones are highly flammable.
Amorphous solids are not crystalline. They do not have a definite shape or a sharp melting point. They are amorphous and semi-crystalline. An amorphous solid has a definite melting point. Amorphous solids are amorphous. They do not have a cleavage.
The main difference between amorphous and crystalline solids is the degree of cleavage. The former has a regular arrangement of particles while the latter has a disorderly arrangement. As a result, a crystalline solid is a solid that can cleave along specific points. Amorphous solids have a definite melting point but have an amorphous part.
Amorphous solids are the same as crystalline ones, although they may have different names. Amorphous solids have no crystalline structure and are amorphous. They can be molded into shapes and used as amorphous plastics. Regardless of whether they're amorphous or crystalline, they are used for many different purposes in our daily lives.
The main difference between amorphous and crystalline solids is that the former have no ordered structure, while the latter have an ordered structure. Crystalline components are held together by uniform intermolecular forces, while these forces vary in amorphised solids from one atom to another. [Sources: 1, 10]
Cut of a crystalline solid that represents a clear cleavage in which the surface cuts at an angle characteristic of a crystal. Crystalline solids have a certain shape and are arranged of ions, molecules and atoms in a three-dimensional pattern called a crystal lattice. A solid body, in which the components of matter are arranged and organized in a certain way, is contained in a crystal, and its structure, the crystal, has a certain geometry. [Sources: 10, 11]
In terms of how crystalline solids break when they show what geologists call cracks, this means that they tend to break along certain levels of their crystal structure. The regular internal chemical structure causes crystalline solids to have a clearly defined melting point. [Sources: 13]
Amorphous solids tend, in contrast to crystalline solids, to soften over a wide temperature range without a clearly defined melting point. Particles of such solids lack order in the arrangement of internal structures (Figure 1). In view of this disturbance in the arrangement, these solids are referred to as supercooled liquids. [Sources: 1, 9, 12]
In the physics of the condensed matter and materials science, an amorphous (Greek: a-morphous) form forms non-crystalline solids, solids which lack the long-term order characteristic of crystals. Amorphous solids retain a temperature below their melting point for a longer time period, and the component molecules (atoms and ions) rearrange in a more ordered crystalline form. [Sources: 7, 12]
An amorphous material has no internal structure and consists of interconnected building blocks. These blocks resemble the basic structural units found in the corresponding crystalline phases of the same compound. [Sources: 7]
Solid materials can be divided into two different types: crystalline materials and amorphous materials. In nature, amorphous material is an atomic length scale of short order without chemical bonding (see structure of liquid glass for more information on non-crystalline material structures). It is common to refer to amorphous materials as glass due to the transition temperature between frozen solid and supercooled viscous liquid. [Sources: 4, 7, 8]
The structure of amorphous solids was described as crystal-like with short-range molecular arrangements and a lack of long-term order. In the immediate vicinity, the molecules of the material do not differ significantly from those of the crystals, with similar numbers and distances to the nearest neighbors, but they lack the translational and Ori symmetries that characterize crystals. [Sources: 8]
The extent of structural similarity between amorphous phases and their corresponding crystalline polymorphs has not yet been quantified. We describe a new approach to quantitative measurement of the structural similarity of amorphous and crystalline phases applied to the case of TIO-2. The ideal crystal is defined by the periodic translation of a single cell unit, but the lack of inherent periodicity makes such an approach impossible for amorphous phases except to a considerable extent in the short- and medium-term orders. [Sources: 2]
Although rubber is an amorphous solid, it has other physical properties. A snapshot of the generated liquid amorphous TIO and A-TIO models is shown in Figure 1. Similar to crystals, the most important structural characteristics of these solids are polyhedric units connected by vertices, edges and surfaces. [Sources: 2, 5]
When we talk about solids, we often think of the position of atoms, molecules or ions (which are fixed in space) and their movement, which is particularly important for liquids and gases. With few exceptions, particles consisting of solid materials (ionic, molecular, covalent or metallic) are held in place by strong attractions. With few exceptions in this respect, particles can be part of any solid material. [Sources: 3, 12]
On the good side of anisotropic solids, the term refers to a well-ordered internal structure with a uniform attraction to the crystal lattice. It describes the true properties of a solid with a far-reaching order and rigid structure. [Sources: 10]
They are characterized by a rigid structure of molecules in which the ion atoms are ordered or disordered. Crystal defects In crystalline solids, the atoms of the ion molecules are normally arranged in a certain repetitive pattern, but occasional defects may occur outside this pattern. For example, ionic ions and ionic bonds are brittle and conduct electricity in liquids but not solids ; high melting point (NaCl, Al 2 O 3 ), metallic atoms and electropositive elements with metallic bonds are glossy, malleable and ductile and conduct heat and electricity with variable hardness and melting temperature (Cu, Fe, Ti, U ). Covalent networks of atoms and electronic elements with covalent ENT - bonds are not conductive and have. [Sources: 9, 10]
This arrangement leads to the categorization of amorphous and crystalline, which this article will unfold with the most important differences between the two terms. Glass is often referred to as a supercooled liquid or solid. [Sources: 5, 10]
Solid crystallines are amorphous and exhibit a short-range atomic order. The atomic positions of crystals exhibit a property known as the long-range order, i.e. The translational periodicity of positions that repeat themselves in space at regular intervals (Figure 2a). The definition of the short-range order is a consequence of the chemical bond between the atoms responsible for clumping together the solid. [Sources: 0]
It can be helpful to imagine the distribution of states with different vibrational energies as analogous to the distribution and vibrational energy of states in liquid water in the presence of hydrogen compounds. [Sources: 6]
##### Sources #####
[0]: https://www.britannica.com/science/amorphous-solid
[1]: https://pediaa.com/difference-between-amorphous-and-crystalline-solids/
[2]: https://pubs.acs.org/doi/10.1021/acs.jpclett.8b01067
[3]: https://www.vedantu.com/chemistry/difference-between-crystalline-and-amorphous-solid
[4]: https://chembam.com/definitions/crystalline-vs-amorphous/
[5]: https://courses.lumenlearning.com/cheminter/chapter/amorphous-solids/
[6]: https://www.spectroscopyonline.com/view/why-are-raman-spectra-crystalline-and-amorphous-solids-different
[7]: https://en.wikipedia.org/wiki/Amorphous_solid
[8]: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7245478/
[9]: https://opentextbc.ca/chemistry/chapter/10-5-the-solid-state-of-matter/
[10]: http://www.differencebetween.net/science/difference-between-crystalline-and-amorphous/
[11]: https://www.askiitians.com/iit-jee-solid-state/amorphous-and-crystalline-solids/
[13]: https://study.com/academy/lesson/how-crystalline-solids-amorphous-solids-differ.html
[14]: https://www.chem.fsu.edu/chemlab/chm1046course/solids.html
