Atomic Layer Deposition (ALD)
What Substrates are Used for Atomic Layer Deposition?
We have a large selection of wafers for your ALD project. Please end us your specs and quantity today!
Get Your Quote FAST!
What is Electrochemical Atomic Layer Deposition (ALD)
A newer approach to atom layer deposition is electrochemical atomic layer deposition (ALD). This technique allows for the development of a variety of new materials and nanofilms, which have a variety of applications. Infrared detectors, photovoltaic panels, and thermoelectric devices are just a few examples. These materials are created through the ALD process. However, many other applications are possible as well.
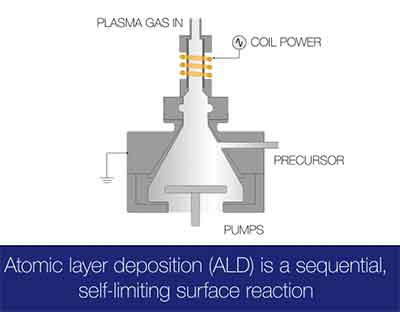 The basic principles of atomic layer deposition are well understood. The atom-by-atom process involves exposing two gaseous species to a surface. These gases are called precursors, and are never present in the reactor simultaneously. The precursors are inserted sequentially, and do not overlap. As a result, they react with the surface in a self-limiting way. The reaction is terminated once all reactive sites on the surface are occupied. The amount of material deposited on the surface depends on the chemistry of the film and the interaction between the surface and the precursor.
The basic principles of atomic layer deposition are well understood. The atom-by-atom process involves exposing two gaseous species to a surface. These gases are called precursors, and are never present in the reactor simultaneously. The precursors are inserted sequentially, and do not overlap. As a result, they react with the surface in a self-limiting way. The reaction is terminated once all reactive sites on the surface are occupied. The amount of material deposited on the surface depends on the chemistry of the film and the interaction between the surface and the precursor.
The ALD process is a controlled and precise method for the deposition of thin films. It is particularly useful for applications that require highly conformal films. The precise control of the ALD process enables new materials and three-dimensional designs. The resulting films are very flexible, and can be used to make everything from solar cells to cell phones. This process is widely used in industry, and is an efficient and safe way to make a variety of different products.
The ALD process is a process that allows scientists to deposit materials at an atomic level. It uses a sequential method to introduce different types of gaseous precursors to a substrate. The first precursor reacts with the first precursor, creating a monolayer on the surface of the substrate. The second precursor reacts with the first one, resulting in the formation of a new monolayer.
ALD is a surface-controlled method of deposition. It results in thin films by forming a monolayer one atomic layer at a time. During a cycle, the reactants and precursors are continuously pulsed. Then, an inert gas is purged between each pulse. In ALD, each molecule of a chemical is exposed to a surface and reacts with it. The ALD process produces a layer of monolayers.
In atomic layer deposition, alternate gaseous species are exposed to the surface and subsequently, form a layer of material on it. The first precursor forms a monolayer on the surface of a substrate, and the second reacts with it to produce a second monolayer. The ALD process is a dynamic process. The temperature of the ALD substrate must be at a lower temperature than the surface of the substrate in order to prevent a reflow of material.
The ALD technique involves alternating precursors and reactants. The electrodes are placed at the surface of the substrate, and the ALD process creates atomic layer structures on the surface. During a cycle, the electrodes are exposed to the reactive precursors. Both of these gases react with the surface in a self-limiting way, resulting in the formation of an alloy. Each ALD cycle is repeated until the desired thickness is achieved.
The ALD process uses a vapor-phase technique called atomic layer deposition. The process involves the introduction of alternating precursors, which are not overlapped and deposited sequentially onto a surface. The AELD process creates a layer of material by applying a uniform film thickness. The alternating precursors and reactants are exposed in a self-limiting way, which stops the reaction once all the reactive sites on the surface are used.
The ALD method involves a series of alternating reactions with gaseous reactants that are deposited onto a surface. This alternating process allows for the deposition of thin films to monolayer thickness and is a versatile method for coating complex objects. It is also capable of producing polymer films with low vapor pressures. This technique is a great choice for high-resolution image capture, as it allows for higher resolution images.
The Difference Between Atomic Layer Deposition and Chemical Vapor Deposition
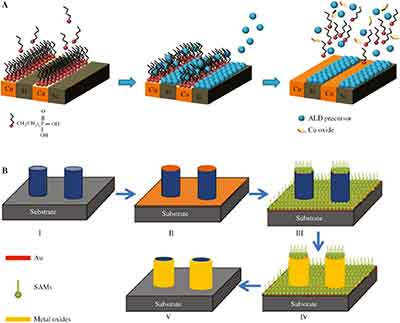 ALD and CVD are two processes that use alternate gaseous species to deposit thin films on a surface. Both processes use atoms and molecules to control the thickness of the deposited layer. Both methods involve multiple pulses of precursors, and no single precursor is present simultaneously in the reactor. The first step in atomic layer deposition involves exposing the substrate to the reactants. After the substrate is exposed to these molecules, they begin to react with the surface. This self-limiting reaction terminates when all reactive sites on the surface are consumed. ALD also provides control over the thickness of the film, and CVD offers a number of advantages.
ALD and CVD are two processes that use alternate gaseous species to deposit thin films on a surface. Both processes use atoms and molecules to control the thickness of the deposited layer. Both methods involve multiple pulses of precursors, and no single precursor is present simultaneously in the reactor. The first step in atomic layer deposition involves exposing the substrate to the reactants. After the substrate is exposed to these molecules, they begin to react with the surface. This self-limiting reaction terminates when all reactive sites on the surface are consumed. ALD also provides control over the thickness of the film, and CVD offers a number of advantages.
Atomic layer deposition is the more accurate and controllable of the two processes, allowing for the deposition of ultra-thin films. This process is also useful for creating three-dimensional designs, and it works well with atomic layer etching. It is the ideal method for creating thin films, as it allows for a controlled thickness without the risk of oxidation.
ALD is a vapor phase process for depositing a thin layer of a material. It involves the sequential introduction of one precursor after another, alternating between them. These two components react with the surface in a self-limiting way. This means that the reaction will stop once all the reactive sites are used. This process can be repeated many times for a desired thickness. You may also combine ALD with another technique, atomic layer deposition.
Atomic layer deposition uses the same technique as chemical vapor deposition, except that the process is based on the vapor phase. In ALD, individual chemical components are introduced to the deposition chamber one at a time. This process provides highly conformal, uniform, and highly controlled films. However, this process can be expensive, and it is slow. As a result, atomic layer deposition is the preferred method for producing thin films.
As both processes use vapor phase chemicals, atomic layer deposition is more complex than conventional vapor phase techniques. ALD is a more complex process than chemical vapor deposition, and it enables the production of thin films with excellent conformality. It can be a more precise method for generating high-quality films, but it is slower than chemical atom-vapor deposition, which is the preferred process for making thin films.
While chemical vapor deposition is a widely used method for creating thin films, atomic layer deposition limits the chemical reactions to the surface of an object. It also results in highly conformal hybrid films. This method is slower than other types of atom-layer deposition. The atomic layer deposition of thin films is slower than the chemical vapor deposition. ALD requires a pure substrate.
Compared to chemical vapor deposition, atomic layer deposition is much more effective for creating ultra-thin films. In addition, it is easier to control the growth rate of films with aLD. In contrast, in a CVD process, a coating is applied over an atom layer. Afterwards, the film is cured. During an ALD cycle, a precursor is added to the surface.
ALD is a type of chemical vapor deposition that allows for extremely thin films. This process is best suited for high-quality, conformal films. ALD is also faster than CVD. It is often advantageous for high-quality, uniform films. The cost of atomic layer deposition is much lower. If you need to produce thin layers, a single-component ALD method is more efficient.
The difference between atomic layer deposition and chemical adsorption and chemical vapor deposition is important for determining the best process for your application. In addition to controlling the thickness, atom-layer deposition allows for surface-controlled, self-limiting films. Both techniques are used to make electronics. ALD produces extremely high-quality, conformal, and uniform films. It also is faster than CPD.
