I am a physics undergraduate at Durham University and am designing an on-chip microwave resonator for my master's project. I am enquiring about the services University wafers offer, which could be used to fabricate this. If this is something you can do, my supervisor would be interested in getting the designs made, provided the production is not too expensive. My basic design is shown below (a CAD file or similar can be produced ready for manufacture). The substrate (green) I am hoping to use is Calcium Flouride (CaF2) and the metal (orange) can be either Gold or Copper. The dimensions I am hoping to use are: t = 0.4 microns (minimum), h = 0.5-1mm, S = 0.35mm, W = 0.15mm, g = 0.15mm Is this something you can make by either sputtering or thermal evaporation? If the values of h, W, S, g are too small/ large for your 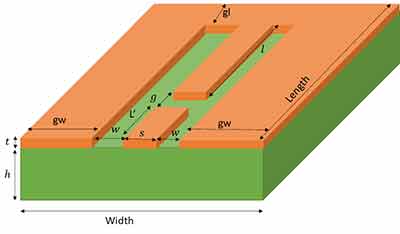 manufacturing process, I can change them within some limits. What are the precisions of these values for your process? Essentially, I want to know if something like this can be made using your facilities and if so, how would it be made? I understand this is a very busy time, so any help on this would be much appreciated. If this is something you can do, I can provide more design details and we can discuss prices/ delivery etc.
manufacturing process, I can change them within some limits. What are the precisions of these values for your process? Essentially, I want to know if something like this can be made using your facilities and if so, how would it be made? I understand this is a very busy time, so any help on this would be much appreciated. If this is something you can do, I can provide more design details and we can discuss prices/ delivery etc.
My design is not finished yet, but the typical ranges of values for the substrate dimensions are: height = 0.5 - 1mm, width = 10-14mm, length = 15-30mm. If you want some numbers to quote now: I'd say use height = 1mm, width = 12mm, length = 24mm. However, I can change some of these dimensions in my design if necessary for fabrication. Is this something which can be done?
What are Calcium Fluoride CaF2 wafers? These are crystals of calcium fluoride that are used to produce optical  components. These devices include thermal imaging systems, spectroscopy, telescopes, and excimer lasers. These materials are transparent over a wide spectrum of frequencies and their low refractive index makes them ideal for a number of optical applications. In addition, the material is highly insoluble in water, making it convenient to process.
components. These devices include thermal imaging systems, spectroscopy, telescopes, and excimer lasers. These materials are transparent over a wide spectrum of frequencies and their low refractive index makes them ideal for a number of optical applications. In addition, the material is highly insoluble in water, making it convenient to process.
Calcium Fluoride is a naturally occurring mineral that is grown in the vacuum Stockbarger technique. Usually, the crystal for infrared use is mined from a deposit, which decreases the cost of production. The absorption band is 300nm, which is why it is called Fm3m. The unit cell is a clear pane of calcium fluoride.
It is produced as a thin film of Calcium Fluoride. This material has a high band gap, a high dielectric constant, and a high laser damage threshold. Additionally, the material is extremely durable in normal atmospheres, which makes it a good material for optical components. Furthermore, the crystal is made with a single-crystal optical quality. If you are looking for a crystal, calcium fluoride may be the right choice for you.
When growing crystals, it is important to remember that the properties of the material are crucial. Besides the purity, calcium fluoride wafers have other properties. For instance, they can be used for laser applications. Their high transmission range, refractive index homogeneity, and laser-damage threshold make them ideal for optical components. Unlike other optical components, CaF2 wafers can be fabricated from high-purity materials. The materials can be made into various types of electronic equipment.
There are several types of Calcium Fluoride wafers. One of the most common is the regent grade, which is also called regent grade. It has a high transmission range and is also resistant to laser damage. It can be etched to make optical components and has excellent IR characteristics. Its refractive index changes over time and depends on the temperature of the substrate.
The optical properties of calcium fluoride wafers vary from product to product. The primary characteristic of a CaF2 window is the high light transmission range. In addition to this, it has a high laser damage threshold and a broad transmission range. Its low density makes it ideal for optical components. Its excellent machinability makes it a desirable component. The other main characteristic is its low thermal conductivity, which is important for many applications.
Infrared technology uses a wide range of wavelengths. To increase the range of infrared light, this material can be patterned with a special technique. When a laser passes through the material, it causes the material to absorb a light in the wavelength that it emits. When a laser is used to cool a spacecraft, it has high thermal conductivity.
Despite being a common optical material, it can be expensive to produce. For infrared applications, it is necessary to obtain an ultra-thin, low-cost substrate. These wafers have an ultra-low melting point and a high melting point, which makes them very expensive. Moreover, it's important to select a material with good optical properties, so it can withstand a wide range of temperatures.
Although calcium fluoride is relatively inexpensive to produce, the cost of natural fluorite is still very high. This is why it's important to choose a high-purity material. It is more cost-effective and more reliable. A low-cost crystal is an excellent option for infrared applications. A good quality one will have very high absorption bands. The other advantage of calcium fluoride is that it's also more stable than its counterparts.
The price of Calcium Fluoride varies according to the quantity and configuration. Depending on the application, these materials are useful in many applications. Using them in photovoltaic cells, for example, is particularly advantageous. They're inexpensive, durable, and environmentally friendly. These materials can also be used to produce photovoltaic panels and solar cells. They can also be used to create lasers and other electronic devices.
Calcium Fluoride (CaF2) Windows
Below are just some of our CaF2 substrates that we have in stock:
- (100), 10x10x 0.5 mm 2 Sides polished
- (100), 10x10x 0.5 mm 1 Side polished
- (100), 10x10x 1.0mm , 1 Side polished
- (100), 10x10x 1.0mm , 2 Side polished
- (111), 10x10x 0.5mm , 2 Side polished
- (111), 10x10x 1.0mm , 1 Side polished
- (111), 10x10x 1.0mm , 2 Side polished
- (111), 1"x1.0 mm , 2 Side polished
- single crystal for evaporation, purity >99.995%, 5x5x5 mm as cut
Sputtering Depostion
Clients use the following CaF2 specs to sputter in the TIFR lab.
Calcium Fluoride Crystal Substrates
- CaF2, (100), 10x10x 0.5 mm 1 Side polished QTY : 10 no
- CaF2, (111), 10x10x 0.5mm , 1 Side polished QTY : 10 no
- CaF2, (100), 2 inch dia x 0.5 mm 1 Side polished QTY : 1 no
- CaF2, (111), 2 inch dia x 0.5mm , 1 Side polished QTY : 1 no
CaF2 for FTIR
Researchers use CaF2 for Fourier-transform infrared spectroscopy (FTIR). This method obtains the infrared spectrum of absorption or emission of a solid, liquid or gas. An FTIR spectrometer simultaneously collects high-spectral-resolution data over a wide spectral range.
Material Safety Data
MSDS avaialable upon request.
Can Fajan's Rules Explain Why Calcium Fluoride Has a Lower Melting Point Than Calcium Oxide?
Can Fajan's rules explain why Calcium Fluoride has a lower melting point than calcium oxide? The answer is yes, but the process of comparison is not straightforward. The difference in the compounds is due to the charge distribution. In general, a compound with a higher charge has a higher melting temperature than a compound with a lower charge.
According to Fajan's rules, a molecule's covalent character depends on its electrostatic force and effective nuclear charge. The latter, which is the most important factor, is related to the size and relative charges of the cation and the anion. As the size increases, the effect of oppositely charged ions is lessened. Thus, the melting point of CaF2 is lower than that of its cousin, iodide.
An electronegativity difference between a cation and an anion is a good predictor of the type of chemical bond. A greater difference in electronegativity between two molecules means that the bonds are more polar. Therefore, Linus Pauling proposed an empirical relationship between percent ionic character and the difference in electronegativity between the two molecules. This is shown in the red curve below.
Can Fajan's rules explain why CaO has a lower melting point than CaF2? This question is controversial but is a common question among chemists. For example, the difference between the two compounds in terms of their molecular structure is the primary factor behind the difference between their melting points. If a substance has a low molecular weight, it will have a lower melting point than one with a high molecular mass. If it is larger, it will be more polar.
Can Fajan's rules explain why CaO has a lower melting point than CaF2? In addition to being covalent, CaF2 is also an ionic compound. This means that it contains a cation that is conjugated with a base, while a cation that has a high charge has a lower charge than a cation.
The rules based on the Effective Nuclear Charge and Electronegativity can explain why CaF2 has a lower melt point than CaO. By comparing the ionic nature of the two molecules, they can understand why the former is warmer than the latter. And the answer to this question can be found in a number of other ways. For example, an ionic compound contains more potassium than CaF2.
As a weak acid, CaF2 has a lower melting point compared to its sister, the latter has a higher melting point than the former. An ionic compound is an acid that has a smaller melting point than CaO. An ionic compound can have a lower melting temperature than a cation with a higher charge.
The chemical properties of CaF2 are a result of its ionic charge. In contrast to calcium, strontium ions are larger than calcium and fluorine. This means that $ceCaF2$ will have a higher bond strength than CaO. A higher bond strength means more energy is required to break it. It is thus essential to understand the differences between the two compounds in order to understand how they differ.
In general, ionic charge can be determined through the electrochemical equation. Hence, the electronegativity of a compound will help you determine its ionic character. The greater the difference, the higher the degree of polarity of the bond. If the difference is greater, it indicates that the bond is less ionic. If it is smaller, the chemical reaction will not occur.
An ionic compound is an ionic compound. If the ionic compounds were liquids, they would not be polar. This is because ionic compounds have a low melting point. The opposite is true when the ionic charge of a compound is large. This results in a higher polarization. Moreover, the difference between the ionic charge and the ionic capacity of a substance is smaller.
What is Polycrystalline Calcium Fluoride?
You may be wondering what is Polycrystalline Calcium fluoride (PCF)? This article will provide you with the basics of this important material. You will learn about the uses for PCF, as well as how it is manufactured. The benefits of PCF are also discussed. This mineral can be used in the manufacture of a number of different products. For example, it is used to create a wide range of plastics.
Spectroscopic CaF2 Windows
Spectroscopic CaF2 windows have excellent transmission properties from 130nm to 10um and have numerous applications in a variety of fields, including white light generation. These windows are highly efficient at transmitting light, and their thickness ranges from 0.5 to 100 mm. They are available in three different grades, including regent grade, Eximer grade, and Raman grade, and are designed to work at specific wavelengths.
Spectroscopic CaF2 windows offer several advantages over other materials. Because they exhibit excellent transmission in the visible and ultraviolet, they can also be used in laser applications. Their low density, wide transmission range, and excellent machinability make them ideal for optical components. Furthermore, their low thermal conductivity make them a highly cost-effective material for a variety of applications.
Spectroscopic CaF2 windows were created by scientists at Sandia National Laboratories, a multi-mission laboratory run by the U.S. Department of Energy and the National Nuclear Security Administration. These windows provide an ideal optical window through which researchers can study irradiation processes. Detailed information about the irradiation path of CaF2 has now been published.
Using spectroscopic techniques, researchers have studied the effects of 100 keV Tb ion implantation on polycrystalline CaF2. They observed that the ionic state of Tb is not affected by coexistence of the secondary phases. This results in improved antireflective coatings and down-conversion of light. The spectral range of the conversion is also expanded, leading to greater conversion efficiency.
Another method for making optical windows of polycrystalline calcium fluoride is through the use of ion beams. By using a grazing incidence, 100 MeV ions irradiated on CaF2 can create novel ion-tracks. This allows researchers to track the forces to the surface in an atomized state. The resulting ion-tracks, which are visible under spectroscopy, reveal three distinct parts. During the first stage of the process, a fast heavy ion opens a groove that spans 100aEUR
Vacuum Ultraviolet Scintillator
A new spectrophotometer for measuring the transmittance of Polycrystalline Calcium fluoride crystals has been developed. This new device offers several advantages over the conventional method of direct measurement using F2 or excimer lasers. Among these benefits, its ease of use, accuracy, and low cost make it an excellent choice for internal transmittance measurements. This technology is applicable for measuring light wavelengths and transmittance of many materials, including polycrystalline Calcium fluoride.
The light emitting element of the present invention is a metal fluoride crystal represented by the chemical formula LiM1M2F6. Li includes six Li and M1 and M2 represent alkaline earth metals and metal elements. Eu2+ is at least 0.02% by mole in this material. The resulting product efficiently volatilizes without remaining trapped in the polycrystalline Calcium fluoride crystal.
This chemical compound can be highly sensitive to moisture above 500degC. Because of this, barium fluoride is not ideal for a vacuum environment. However, it is a good candidate for electrochromic filters. It is also highly resistant to X-rays and is used in a wide variety of applications. It is useful in research and medical applications and can be used in a wide range of environments.
The chemical composition of calcium fluoride crystals is an important factor in the quality of the material. The material can be produced in many ways, including by hot-pressing. When the polycrystalline material is heated, it recrystallizes and retains its optical properties. However, the heat and force involved in manufacturing it makes it prone to absorption bands and may not be suited for certain applications.
Lead Conversion Into Polycrystalline Calcium Fluoride (CaF2)
Polycrystalline calcium fluoride is an important component of a variety of ceramic materials, including ceramic tiles, sand, and marble. The process of lead conversion into polycrystalline calcium fluoride involves the chemical reaction of lead and calcium fluoride. During this process, the lead is oxidized to a neutral salt, which is called calcium fluoride. CaF2 nanoparticles contain Yb,Er, and Tm.
Fluoride exposure affects the physiochemical and structural properties of bone. It also impacts calcium control in rabbits. These are just a few of the detrimental effects of fluoride, but the effects of Fluoride on bone are well documented. The findings from the present study will help guide future research into the toxic effects of fluoride on human health. Calcium supplements, in particular, are essential for a number of reasons.
Light Transmittance
In order to investigate the light transmittance of Polycrystalline Calcium fluorides, the researchers irradiated the samples with a F2 laser and then measured their spectral transmittance. To accomplish this, the crystals were optically ground with diamond grains to prepare them for the F2 laser. The wavelength of the laser used in this study was 890 nm. As such, the calcium fluoride crystals showed very high light transmittance.
In order to improve the light transmittance of the Polycrystalline Calcium fluoride crystal, the material is heat treated to reduce the thermal stress. This crystal is then cut and processed to form an optical member. The light transmitted by the optical member is approximately 82%. It should be noted that the optical performance of this material decreases with increasing distance from the light source. This is because it loses 1% of its intensity with every 10 mm of length.
The internal transmittance of Polycrystalline Calcium fluorides was comparatively high, although the spectroradiometer's accuracy was poor. The light transmitted through the material decreased with decreasing thickness, and the internal reflectance was 99.5% versus 100%. Both samples showed very similar internal transmittance, and the difference was only 0.1% for the Comparative Example. This resulted in a highly inconclusive comparison.
Crystaltechno Ltd. is a company that manufactures crystals and optical components for use in a variety of applications, including medical technology, laser research, and aviation. Its products are used in special equipment, and their light transmittance spectra are extremely broad. The company is currently developing a new range of optical components, which include laser lenses and other optical components. This allows it to serve as a versatile, low-cost alternative to other types of materials.
What is Calcium Flouride's Melting Point?
The process of making Polycrystalline Calcium fluoride begins with a preprocessing furnace. The furnace is maintained at a vacuum of 10-3 to 10-5 Pa and gradually increases the temperature until it reaches the melting point of calcium fluoride. Then, the material is placed into the growth crucible, where a single crystal is grown. As the growth furnace heats up, the desorbed gas in the preprocessed material is mixed with the growing crystal.
This process requires a vacuum furnace. The pressure inside the furnace is slowly increased to maintain the high vacuum. The melting point of polycrystalline Calcium fluoride is around 700 degrees Celsius. At a temperature higher than this, the carbon compounds on the surface of the powder begin to decompose. The upper limit of the furnace is 1350 degrees C. During this process, the temperature is monitored to ensure that the final product is stable.
The spectral absorption coefficients of calcium fluoride were determined using a single-beam ir spectroradiometric system. The wavelength range was 2-12 microns, and the temperature was changed from 500 degrees C to 600 degrees C. The finalized ceramics were characterized by X-ray diffraction, scanning electron microscopy, and Fourier transform infrared spectroscopy.
The final temperature of polycrystalline calcium fluoride is approximately 100 degrees above the decomposition temperature. The crystallization process is a complicated process. A mixture of calcium fluoride powder and a scavenger is mixed with the starting material. Then, the material is placed in the growth furnace. The temperature inside the furnace gradually rises until the desired melting point is reached. The crystals are then formed.
What are The Benefits of Calcium Fluoride Wafers?
Choosing the right dental care for your teeth has never been easier thanks to the variety of fluoride products that are available in the market. These products come in the form of wafers, gels, and mouthwashes. Some are cheaper than others, and some are more stable than others.
Calcium fluoride is cheaper than natural fluorite
Using calcium fluoride in water supplies has been a controversial practice. There are many arguments as to why this is a bad idea. Some argue that the calcium ions in the water are toxic and should be removed. Others claim that the fluoride is harmless and useful for teeth.
The truth is that fluoride is a mineral that is used in a variety of industries. It is also used for jewelry and ornamental carvings. It is also used in glass manufacture. The manufacturing of these materials requires a high purity of calcium fluoride.
There are two types of fluoride; natural and industrial fluoride. Natural fluoride is naturally occurring and is found in some water supplies. Industrial fluorides are made from fluorine and fluoride. Industrial fluorides are fully absorbed by the body. The difference between natural and industrial fluoride is that the natural types contain a natural metal cation. Industrial fluorides contain a synthetic fluoride ion.
Industrial fluoride is used for drinking water and in many industrial applications. It is also used for dental treatment. The crystalline form of fluorite contains a calcium ion and is a valuable mineral in many industrial applications.
Fluorite is also known as murrina in the Latin language. It is found in various areas of the world including China, Argentina, Tanzania, South Africa, Canada, and the United Kingdom. The mineral has a variety of colors, including reddish purple, white, and brown. The color is determined by the substitution of calcium for other elements in the crystalline structure.
Calcium fluoride is often used as a window material for infrared and ultraviolet wavelengths. It is also used for semiconductor manufacturing. It has a non-linear refractive index at high power densities. It also has deep coloration due to impurities. It is used to make optical elements that help reduce chromatic aberration.
Natural fluorite has a variety of uses including smelting flux and optical components. It is used as a precursor for HF. It can also be used to make glass and enamels. It is not used widely as a semiprecious stone.
The production costs of mining fluorite are much higher than those of naturally occurring fluorite. Most of the mining took place in underground mines at depths of more than one thousand feet.
Calcium fluoride is more stable than its counterparts
Unlike other fluorides, calcium fluoride is relatively stable and durable in normal atmospheric conditions. This is beneficial for applications in photovoltaic cells and lasers. Its optical quality, high transmission range, and low laser damage threshold makes it a good choice for optical components.
Calcium fluoride is a white insoluble mineral. It is produced by adding hydrogen fluoride to calcium carbonate. It has a refractive index of 1.361.42, a high dielectric constant, and a high band gap. It is a common substance used to make optical components and electronic devices. Calcium fluoride can be produced in thin films that are transparent over a wide range of frequencies. It is an excellent choice for infrared applications. Its crystal thin film has high dielectric strength and a high band gap.
In addition, calcium fluoride is useful in producing photovoltaic panels. The regent grade has excellent IR characteristics. It is resistant to laser damage, but its refractive index changes with time. The refractive index of the regent grade depends on the temperature of the substrate.
It is difficult to make high-purity calcium fluoride with the methods used in prior art wastewater treatment systems. Although this process is able to reduce by-produced 2.4% H2SiF6, it results in a 10 to 15% loss of cost when compared to the pure component. Moreover, the process does not produce uniform results.
A better recovery technique is needed to achieve high purity. The process must also be able to achieve reactivity percentages that are almost 100%. This requires a long time to complete the reaction, which increases the equipment scale.
The calcium fluoride system consists of a reaction tank R1 and a thickener R2. The thickener separates the liquid phase from the solid phase. The solid phase is then treated with 5% hydrofluoric acid. The liquid phase part of the etchant is then removed and the powder calcium carbonate is added to the residual fluorine. The process is repeated until the desired purity is achieved. The results are shown in Tables 3 and 4. The reactivity percentage of the regent grade is very high.
Calcium fluoride wafers take long times to settle
Compared with other fluoride materials, Calcium fluoride wafers are relatively inexpensive to produce and have excellent lifetime stability. They are used in laser applications as well as in photovoltaic cells. Calcium fluoride is particularly useful in the DUV range of wavelengths. It is also durable in normal atmospheres. It is highly transparent, with high transmission ranges and band gaps. It is used for prisms and lenses.
Calcium fluoride is also a good candidate for the construction of laser optics, because it has a wide range of transmission. It has a high damage threshold, and is stable in both dry and wet environments. Its refractive index is also high. It can be used in a range of wavelengths from 0.15 um to 9 um.
It is a relatively cheap material to produce, and it is very durable at high temperatures. It is a good choice for high power laser optics, especially in the IR less than 6 microns. It can also be used for excimer systems, since it has a high dielectric constant and a low absorbtion.
The purity of Calcium fluoride can be improved by adding fluorosilicic acid, which is produced during the etching process. A high purity Calcium fluoride wafer can be made using a counterflow technique.
Counterflow is a method that requires the simultaneous flow of a calcium carbonate and etchant. This technique can achieve 100% recovery and high purification of the calcium fluoride. However, this method involves large scale equipment. Its advantages are that it eliminates waste and allows for the reuse of fluoride. It is also effective in producing high purity fluorite, which is cheaper and more reliable.
In addition to the recovery process, there are several other processes that can be used to improve the purity of the calcium fluoride. These include the addition of a chemical equivalent, such as a fluorosilicic acid, and an etching assistant, such as acetic acid. Adding a surfactant can also help.
In addition, it is important to minimize the amount of unreacted calcium carbonate. This can be achieved by using a counterflow HF5% hydrofluoric acid treatment. The ratio of the recovered calcium fluoride to the treatment amount of solution can be 4 to 10/100.
Calcium fluoride causes skeletal fluorosis
Symptoms of crippling skeletal fluorosis are severe, painful, and affect mobility and muscle wasting. This type of bone disease is a result of the accumulation of excess fluoride in the body. It can cause painful calcification of ligaments and joints. It can also lead to neurological damage due to spinal cord compression.
Fluoride is a naturally occurring mineral that is found in rocks and water. It is also present in certain foods. The amount of fluoride present in the body depends on how much fluoride is ingested. Fluoride can be detrimental to the health of both adults and children. In order to prevent skeletal fluorosis, it is important to eliminate sources of fluoride from the diet.
Several studies have shown that high doses of fluoride can cause gastrointestinal irritation, stress fractures, and abnormal bone mineralization. The US Environmental Protection Agency (EPA) recommends a maximum of two milligrams per liter (mg/L) for children. However, this recommendation is not enforceable by law.
The extent of long-term fluoride toxicity depends on the age, gender, and sex of the individual. In addition, the amount of calcium ingested is also a factor in the outcome of fluorosis.
The present study investigated the effects of fluoride toxicity on hormonal parameters, bone microstructure, and histological parameters. Fluoride-induced bone lesions are characterized by increased bone mass, osteosclerosis, and exostosis formation.
Fluoride toxicity can be prevented by taking a fluoride-free diet, eliminating sources of fluoride, and avoiding water with high fluoride levels. Moreover, exercise can help to reduce toxins from the body. A fluoride-free diet should also include organic foods. This diet should include animal products, fresh fruits, and vegetables.
There are several therapies that can help to reduce bone loss. Nevertheless, fluoride therapy probably cannot restore bone resorption. Calcium supplementation has also been found to be effective in ameliorating some of the hazardous effects of fluoride.
A low fluoride dose has been found to reduce the incidence of fractures. However, the use of fluoride therapy may be less effective in patients with substantial trabecular connectivity. Moreover, fluoride may affect bone mineral metabolism.
CaF2 Windows UV Grade
Calcium fluoride (CaF2) windows are transparent optical elements made from single-crystal calcium fluoride they are used in a variety of applications, including:
- UV spectroscopy: CaF2 windows are used as UV-transmitting windows in spectrometers and other optical instruments. They are beneficial for applications in the UV-VIS-NIR region, where they have high transmission and low absorption.
- Laser optics: CaF2 windows are used as protective windows in laser systems, where they can withstand high power densities and high repetition rates.
- Infrared (IR) imaging: CaF2 windows are used in IR imaging systems as they have high transmission in the mid-IR region and low absorption.
- X-ray imaging: CaF2 windows are used in x-ray imaging systems because they have high transmission and low absorption in the soft x-ray region.
- Astronomy: CaF2 windows are used in telescopes and other astronomical instruments as they have high transmission in the near-IR region and low scattering.
UV-grade CaF2 windows are made from high-purity calcium fluoride and are carefully polished to minimize surface defects and scattering. They are designed for use in UV applications where high transmission and low absorption are critical.
CaF2 Windows IR Grade
Calcium fluoride (CaF2) windows are transparent optical elements made from single-crystal calcium fluoride and are used in a variety of applications, including:
- Infrared (IR) spectroscopy: CaF2 windows are used as IR-transmitting windows in spectrometers and other optical instruments. They are useful for applications in the mid-IR region, where they have high transmission and low absorption.
- Laser optics: CaF2 windows are used as protective windows in laser systems, where they can withstand high power densities and high repetition rates.
- Infrared (IR) imaging: CaF2 windows are used in IR imaging systems as they have high transmission in the mid-IR region and low absorption.
- X-ray imaging: CaF2 windows are used in x-ray imaging systems because they have high transmission and low absorption in the soft x-ray region.
- Astronomy: CaF2 windows are used in telescopes and other astronomical instruments as they have high transmission in the near-IR region and low scattering.
IR Grade CaF2 Protective Windows for Spectroscopy Applications
Calcium fluoride (CaF2) windows are transparent optical elements made from single-crystal calcium fluoride they are used in a variety of applications, including:
- UV spectroscopy: CaF2 windows are used as UV-transmitting windows in spectrometers and other optical instruments. They are beneficial for applications in the UV-VIS-NIR region, where they have high transmission and low absorption.
- Laser optics: CaF2 windows are used as protective windows in laser systems, where they can withstand high power densities and high repetition rates.
- Infrared (IR) imaging: CaF2 windows are used in IR imaging systems as they have high transmission in the mid-IR region and low absorption.
- X-ray imaging: CaF2 windows are used in x-ray imaging systems because they have high transmission and low absorption in the soft x-ray region.
- Astronomy: CaF2 windows are used in telescopes and other astronomical instruments as they have high transmission in the near-IR region and low scattering.


 manufacturing process, I can change them within some limits. What are the precisions of these values for your process? Essentially, I want to know if something like this can be made using your facilities and if so, how would it be made? I understand this is a very busy time, so any help on this would be much appreciated. If this is something you can do, I can provide more design details and we can discuss prices/ delivery etc.
manufacturing process, I can change them within some limits. What are the precisions of these values for your process? Essentially, I want to know if something like this can be made using your facilities and if so, how would it be made? I understand this is a very busy time, so any help on this would be much appreciated. If this is something you can do, I can provide more design details and we can discuss prices/ delivery etc. components. These devices include thermal imaging systems, spectroscopy, telescopes, and excimer lasers. These materials are transparent over a wide spectrum of frequencies and their low refractive index makes them ideal for a number of optical applications. In addition, the material is highly insoluble in water, making it convenient to process.
components. These devices include thermal imaging systems, spectroscopy, telescopes, and excimer lasers. These materials are transparent over a wide spectrum of frequencies and their low refractive index makes them ideal for a number of optical applications. In addition, the material is highly insoluble in water, making it convenient to process.