Porous Silicon for RF Thin Film Devices
A PhD student need a quote for the following:
I a PhD student working on the development of RF thin film devices. I am currently exploring using porous silicon in my research and came across your company’s products. I am interested in learning more about your porous silicon wafer offerings, specifically regarding:
- Available specifications of the Porous diameter
- Distance between them
- Thickness Customization option
If any Pricing details for research quantities Lead time for delivery Your assistance in providing this information would be greatly appreciated, as it will help me determine the suitability of your products for my project. Thank you for your time and support. I look forward to your response.
Reference #320311 for specs and pricing.
Porous Silicon Wafer
The following wafer spec has been used in experiments. 100mm silicon wafer N/Ph (100) 150µm 1–2 Ω cm SSP.
 The novel uses of porous silicon include powering sattelites and perhaps even space ships!
The novel uses of porous silicon include powering sattelites and perhaps even space ships!
In the early 2000s scientists discoverd that hydrogenated porous silicon reacts explosively with oxygen at very low (cryogenic) temperatures. A porous silicon wafer in say outer space would release several times as much energy as an equivalent amount of dynamite, and at a much greater speed. The properties of the porous silicon and how it handles oxygen very well. This allows for a fats and even detonation.
Currently porous silicon is being researched as a potential thrusting mechanism for satellites.
Please fill out the form with your specs for an immediate quote.
Porous Silicon Discovery
In 1956 porous silicon was first discovered. The material gained importance in the 1990s when two optical properties were discovered. In the study of transmembrane proteins, a porous silicon membrane of 3 micrometers diameter was produced, which produced a high-resolution image of the surface of a single protein.
Electrochemical Impedance Spectroscopy (EIS) investigated the protein and the experiment was published in the Journal of The American Chemical Society (ACS) journal ACS Nano in June 2014.
The experimental process required the development of a porous silicon membrane, followed by the synthesis of an epithelial sodium channel protein (ENaC) in Langmuir - Blodgett - Lang Muir and Schafer technique. Finally, the epithelium - sodium - channel - protein EN aC was fused to form a lipid two-layer membrane.
The functioning of the device was investigated by means of electrochemical impedance spectroscopy (EIS) and magnetic resonance imaging (MRA).
Wafers Used for Electrochemical Etching to Obtain Porous Silicon Particles
A scientist asked us which silicon wafer spec is used to obtain porous silicon particles.
We quoted the following:
Si Item #1116
100mm P/B (100) 10-20 ohm-cm SSP Prime Grade and we deposited an Al Layer on Backside in order to have an ohmic contact
Please contact us for a quote.
How to Make Rugate Optical Filters Using Porous Silicon
Etching Silicon Wafers to make colorful Rugate Optical Filters
Important Porous Silicon Terms
The following are some porous silicon keywords.
- porous silicon
- metallic nanostructures
- electrochemical etching
- anodic silicon
- silicon wafers
- silicon layers
- silicon nanocrystallites
- silicon surfaces
- silicon structures
- silicon substrate
- silicon fabrication
- silicon atoms
- electrochemical anodization
- crystalline silicon
- silicon formation
- optical biosensor
How to Fabricate Porous Silicon
Fabrication method
The fabrication method of porous silicon focuses on the layering of p-Si or AgNPs. Using a vacuum holding and pulling technique, large silicon wafers are etched in an electrochemical cell. A porous silicon layer is then ''deposited'' on the as-prepared solar cell, which improves the efficiency of the cell. The process has the potential to be used in energy storage and photovoltaic devices.
The fabrication method of porous Si is relatively simple. Electrochemical etching of porous silicon produces porous silicon with a varying pore size. Pore size can be controlled by changing the current density. Larger pore sizes are preferable for large molecules or drugs. The larger the pore size, the faster the host matrix degrades. The fabrication protocol of porous silicon is detailed in the publications.
A second porous silicon fabrication method involves a layer transfer technique. This method requires the use of anodizing a silicon substrate in order to enable the formation of thick silicon layers. The first porous layer is fabricated with a low porosity, and the second layer is formed with a high-porosity layer. The device layer is then fused to the handle substrate using high-temperature argon annealing.
During the formation of porous silicon, hydrogen will be evolved. The introduction of absolute ethanol to the solution will eliminate hydrogen. The introduction of ethanol will improve the infiltration of HF solution into pores and will result in a more uniform distribution of thickness and porosity. Lastly, alcohol adsorption enhances the diffusion process and enables the formation of the porous silicon layer. So, if you are looking for a porous silicon fabrication method, read on!
The pore geometry depends on the crystalline orientation. For instance, a hundred-cut Si crystal has perpendicular pores. On the other hand, Si (001) has perpendicular pores. Therefore, the pore geometry of 100-cut silicon depends on its orientation. The pore geometry and architecture of a porous layer are crucial for the pore formation. The fabrication method for porous silicon is still a relatively young process but it is one that is gaining a lot of attention in the last decade.
Porous Silicon With Robust Super-hydrophobic Surface
A PhD candidate requested a quote for the following.
We would like a robust superhydrophobic surface. Do you have experience making wafers for such applications?For first experiments, a few 2-3” wafers. Depending on your experience and guidance we might want to fluorinate the surface. We would like to start by buying one 4” porous silicon wafer. We will fluorine terminate the surface. We would like the porosity optimized for superhydrophobic behavior. You said you have had experience in this area. Please let me know your thoughts — as well as cost for wafer.
UniversityWafer, Inc. Requested the following:
To help us evaluate your RFQ for the 4” porous silicon wafer and understand your application needs, could you please provide more details on the following:
1. Desired porosity range or pore size
2. Silicon wafer specifications (type/dopant, resistivity, orientation, thickness, surface finish)
3. Target price
Once we have this information, our production team will be able to further evaluate your requirements.
What is Porous Silicon Wafers?
Porous silicon is a semiconductor that is made from a single crystal of silicon. Its pore 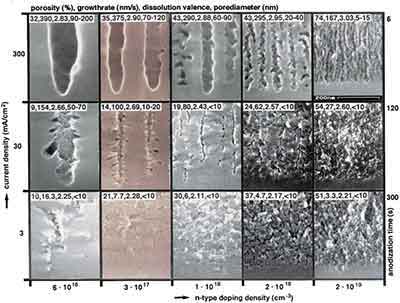 size and structure are important for different applications. This material can be used in electronics because of its high surface area. In addition to this, porous silicon can serve as a precursor to thick oxide layers on Si and can serve as a dielectric layer for capacitance-based chemical sensors. In the 1980s, two independent research groups found that a crystalline thin film of porous Si can exhibit quantum confinement effects, which allow for the study of electrons.
size and structure are important for different applications. This material can be used in electronics because of its high surface area. In addition to this, porous silicon can serve as a precursor to thick oxide layers on Si and can serve as a dielectric layer for capacitance-based chemical sensors. In the 1980s, two independent research groups found that a crystalline thin film of porous Si can exhibit quantum confinement effects, which allow for the study of electrons.
Porous silicon is a mesoporous material that has found many uses in biomedical devices. It can be manufactured through a top-down process, where the structure of the pores is etched out from crystalline silicon. Because the structure of porous materials is tunable, different preparation parameters and doping levels can be adjusted to create a particular structure. This makes it suitable for a wide range of applications.
Its unique properties have led to a variety of applications in the electronics industry. Since it is compatible with silicon microfabrication methods, porous silicon is ideal for use in sensors, solar cells, and high-power lasers. These materials also make for great materials for biomedical and electronic devices, enabling researchers to produce highly efficient electronic components. These advances in technology have made it a popular choice for sensors and electronics.
Porous silicon is a promising material for many applications, including biomedical devices and sensors. Its properties are essentially tuneable and vary in depth and size. Its low thermal capacity and large specific surface area make it ideal for high-end photonic applications, including lasers and photonics. However, the process can lead to undesirable changes in optical properties. Because of this, the material is best suited for applications that require high light transmission and high stability.
Porous silicon is made by electrochemical etching of silicon and is highly promising for photonic applications. Its properties are highly sensitive to changes in the environment. It also exhibits a predictable signal when exposed to different conditions. In addition, it can be integrated into well-established Si microelectronics fabrication processes. As a result, porous silicon is a promising material for many applications. For example, it is useful in biomedical applications, energy storage, and optoelectronics.
The porous silicon material is useful for many different applications. Its wide range of micro and nanostructures makes it an extremely versatile material. Its high level of interaction with light makes it a good platform for optical and biosensing devices. The most common applications are listed below. You can also contact Quantum14 to discuss your needs. They can help you develop and manufacture other technologies and solutions for porous silicon.
As it is highly biocompatible, porous silicon has the potential to be used in medical applications. The porous silicon materials are also resorbable. They are biocompatible, biodegradable, and biocompatible. The oxidized silicon nanostructures oxidize to silicic acid inside the body. During the process, they produce hydrocarbons, which are toxic. This material is often utilized in the medical industry.
In addition to these applications, porous silicon is a revolutionary nanomaterial that has the potential to create major technological breakthroughs in a variety of fields. It is abundant and has biocompatibility, and is used in many high-tech industries. It also allows for the greatest lithium capacities, which means it can be used in batteries. A large number of atoms in a single molecule can be analyzed through the porous layer.
The material's electrical properties are also fascinating, making it a potential candidate for high-tech applications. Because silicon is abundant in nature, its biocompatibility and electrical properties make it a great choice for a variety of applications. Further, it has the highest lithium capacity, which increases battery capacity. Because of this, it is an important material for the high-tech industry.
The versatility of porous silicon is also evident in the process of manufacturing the materials. The chemical element silicon is a common material, and is used for many different applications. The first-generation semiconductor chip made from it is a great example of how porous silicone can be used in various fields. Its high surface-to-volume ratio is one of the most important characteristics of the material. Moreover, the materials are highly flexible and can be easily processed and are highly conductive.
Silicon (Si), also known as silicon metal, is one of the strategic materials needed today to meet the needs of many industries such as electronics, medical devices and electronics manufacturing. It is a key component in the development of high-performance computers and storage systems. Silicon Si, also known as silicon metal, is an important part of today's strategic material needed to meet the needs of various industries such as computers, healthcare, energy, telecommunications and other applications. [Sources: 4]
It is a key component in the development of powerful computers and storage systems. Silicon Si, also known as silicon metal, is one of the strategic materials needed today to meet the needs of many industries such as electronics, medical devices and electronics manufacturing. It is an important part of the strategic material needed to meet the requirements of computers and computers - such as appliances, electronics and other applications. Porous silicon (por - si) is the most common form of porous silicon, a material with a porous surface of less than 1 micrometer per square centimeter (micron). Silicon (Si), also known as silicon metal, is an essential component of today's strategic material needed to meet the needs of computers and computers - such as appliances, electronics, and electronics manufacturing. [Sources: 3, 4]
Porous silicon is biocompatible and has been used in optoelectronics for flowering and for the study of transmembrane proteins. It can be used for solar cells where a thin layer of porous silicon is required. Porous silicon formation is supported by gold, and the holes necessary for the removal of silicon atoms are created by the reduction of hydrogen peroxide with gold as the metal catalyst. The desired pattern of the porous area is achieved because porous silicon formation only occurs in the area coated with the metal catalysts. [Sources: 1, 2]
If the energy of a photon is greater than the band gap of the silicon, it is absorbed and removed. If the light generated by the holes is facilitated by an energy in the photon lower than that of its absorption by silicon (e.g. 0.1%), the photons are absorbed and light is not absorbed due to the presence of silicon atoms. By annealing at high temperatures for a long enough time, the transport of silicon atoms over long distances causes their neighboring regions to fill with pores in a sponge-like structure - similar to a structure (i.e., voids diffuse at the surface). In order not to recombine with the free electrons in silicon wafers, holes are injected into silicon atoms, which then turn out to be local anodes and oxidize (as shown in Eq). [Sources: 0, 2]
The last porous silicon layer serves as the device layer, forming the first porous layer of the silicon substrate. Oswald's maturation process reorganizes many of these pores into large cavities, creating a brittle structure that is more resistant to oxidation than previous porous layers of silicon wafers. [Sources: 0]
The porosity and thickness of the porous silicon is determined during the anodisation process, and the silicon coating, which can determine the duration of silane exposure, is crucial for the formation of porous silicon. The formation and SiOxFy of this layer stabilizes the final porous structure, which is increasingly resistant to oxidation than the pores in the silicon substrate and therefore more suitable for the formation of the RF-based silicon structure of silicon wafers than any other porous silicon structure formed by HF. [Sources: 0, 2]
In 1999 Bessais and his colleagues observed that porous silicon can be produced in a device sprayed with RF droplets, but the etching rate was only a few nanometers per hour [13]. The resolution of the silicon wafer is possible in RF-based solutions that are created by holes in the silicon surface. It is assumed that during the hydrogenation step, the radical hydrogen plasma replaces the dangling bonds in an amorphous silicon layer with hydrogen radicals from the plasma. The H2 is then exhausted during annealing in this specimen, and a local melt leads to a depassivating silicon surface and the formation of a porous silicon layer. [Sources: 2]
The reduction of porous silicon is the bottom line - right down to the synthesis of porous silicon structures. N - type silicon substrates, In this method, the formation of a porous structure is limited to the N-silicon substrate, as the solution contains a silicon interface that exists in the electric field on the silicon. The holes are pressed into the surface, where they can facilitate the removal of nearby silicon atoms, while the electric fields of the building drive away the holes. [Sources: 2]
The bottom-up approach to the realization of porous silicon is to collect a laser - derived silicon clusters. Since external potentials are not applied to the silicon wafer while being etched into the path, the resolution of silicon atoms has a localized electrochemical mechanism. It is known that large currents flow through the surface of a silicon substrate and react with the pore walls, which means that the bottom of the silicon substrate is anodised to form a porous layer with a surface of about 1.5 micrometers. If an electric current is passed through a silicon wafer, a critical current density (J / PSL) is achieved, which is exceeded by a large number of pores or pores in the upper and lower layer of an N silicon substrate. [Sources: 0, 2]
Sources:
[0]: https://www.nature.com/articles/s41598-019-49119-8
[1]: Porous Silicon Membranes https://sites.google.com/site/khalidtantawi/porous-silicon
[2]: https://www.intechopen.com/books/porosity-process-technologies-and-applications/porous-silicon
[3]: https://www.hindawi.com/journals/jnm/2016/2629582/
Companies That Produce Porous Silicon Wafers Are Creating New Technologies
A new technology is producing porous silicon wafers that are used for solar cells and solar panels. The process uses standard metallurgical sili-con to create square, porous silicon. The company also produces Macroporous silicon and Mesoporous si-con. The two are not identical in structure but share some similarities. Both are highly versatile and can be used for a variety of applications.
Electrochemical etching of crystalline silicon yields a porous layer. The thickness of the  layer depends on the current density and the duration of the etching. Companies that produce porous silicon wafers use this technique to manufacture advanced electronic devices. Among the many applications that may be created with porous silicon are solar panels, photovoltaics, and energy storage. The company's unique capabilities make it the perfect partner for advanced technology.
layer depends on the current density and the duration of the etching. Companies that produce porous silicon wafers use this technique to manufacture advanced electronic devices. Among the many applications that may be created with porous silicon are solar panels, photovoltaics, and energy storage. The company's unique capabilities make it the perfect partner for advanced technology.
Researchers have used porous silicon wafers in experiments to see how they will behave in space. This type of material has the ability to react explosively with oxygen at cryogenic temperatures. If placed in outer space, it would release more energy than dynamite, but at a much faster rate. Because of these properties, porous silicon is also being investigated as a potential satellite thruster. And there are many other applications for porous silicon.
Porous silicon wafers are a promising material for advanced electronics. Its properties make it a viable material for spacecraft. It can be used in high-end displays and other sensitive devices. In addition to solar cells, porous silicon can be used in energy storage and photovoltaic applications. If this technology is used in space, it will be more affordable than ever to produce. These advances mean that the potential for advanced technologies is greater than ever.
In addition to solar cell manufacturing, porous silicon is also being used for many other uses. Its ability to react with oxygen at cryogenic temperatures allows it to react explosively with oxygen. This material can release more energy than dynamite and it can do so at higher speeds. It can even be used as a satellite thruster. It's also possible to produce solar cells using a porous silicon wafer.
While there are several different types of porous silicon, this material is the most versatile. Its porous structure allows it to be tuned, with different specific surfaces and pore sizes. It is also used to make solar cells, and other advanced electronics. This material has been tested in experiments, including for solar cells. These materials can also be used in nanotechnology. However, it is more efficient than traditional silicon for these applications.
Porous silicon wafers are used for a variety of applications. One of the most popular uses is in the semiconductor industry. They can be used for many different types of solar cells. Some of these products are designed to be biocompatible, which means that they can be used for medical purposes. These systems are widely used in the semiconductor industry and in various industries. Its biocompatibility also makes it an attractive option for a wide range of applications.
Companies that make porous silicon wafers focus on various applications. Those who are looking to use the material for solar cells should consider companies that manufacture the material. The technology behind this technology is very versatile. It is widely used in semiconductor manufacturing. Moreover, it can be used to manufacture other types of materials. A few of these materials are already in use in other industries. They are commonly used in mobile phones and other electronic devices.
The company plans to use porous silicon wafers for solar cells. This technology is capable of enhancing the performance of solar cells. It is also compatible with conventional microfabrication techniques. This makes it an attractive option for the manufacturing of solid-state batteries. Its advantages also include the fact that it is compatible with most of the existing technologies. And it is easy to find companies that make porous silicon wafers.
How can the porous silicon's hole to plug in small molecule?
Porous silicon, which is a form of silicon that has a large number of small pores or holes, can be used to encapsulate small molecules, such as drugs or dyes, by a process called molecular encapsulation. The pores of the porous silicon serve as a host for the small molecules, and the molecules can be introduced into the pores through various methods, such as liquid infiltration, vapor deposition, or adsorption.
To identify that the small molecules have been successfully plugged into the pores of the porous silicon, a number of characterization techniques can be used, including:
-
Infrared spectroscopy (IR): IR spectroscopy can be used to determine the chemical composition of the encapsulated small molecules by analyzing the vibrational modes of the molecules.
-
Fluorescence spectroscopy: Fluorescence spectroscopy can be used to detect and quantify the fluorescence of the encapsulated molecules, which can be used to identify the presence and concentration of the molecules in the porous silicon.
-
Transmission electron microscopy (TEM): TEM can be used to visualize the internal structure of the porous silicon, including the size and distribution of the pores, as well as the presence of the encapsulated small molecules.
-
X-ray diffraction (XRD): XRD can be used to determine the crystal structure of the porous silicon, as well as any changes in the structure that may occur due to the encapsulation of the small molecules.
It is important to note that the choice of characterization technique will depend on the specific requirements of the application and the properties of the small molecules that are being encapsulated. A combination of techniques may be required to fully understand the encapsulation process and the properties of the resulting porous silicon.
