I am researching the effects of intense radiation on different materials of varying ionization energies. Lately I have only been studying gases but would like to start studying semiconductor materials of varying band gaps. I am interested in getting some semiconductor materials but since I am just going to bombard them with radiation, I was wondering if you had an like scrap material from the dicing or like broken wafers. I dont want to spend over $100 per wafer its just going to get destroyed after about a minute of radiation. Can you let me know if you have any material such as this? I would be needing pieces about 1cm in length or larger of course, preferably the same in width, and thickness should not matter as I am studying surface defects for now.
It would also be ideal to have a variety of materials, Si, Ge, GaAs, InSb, InAs with some undoped, some doped n, some doped p.
Researching Iintense Radiation Effects on different Materials of Varying Ionization Energies
A nuclear engineering graduate student requested a quote for the following:
Reference # 213033 for specs and pricing.
Get Your Quote FAST! Or, Buy Online and and start researching today!
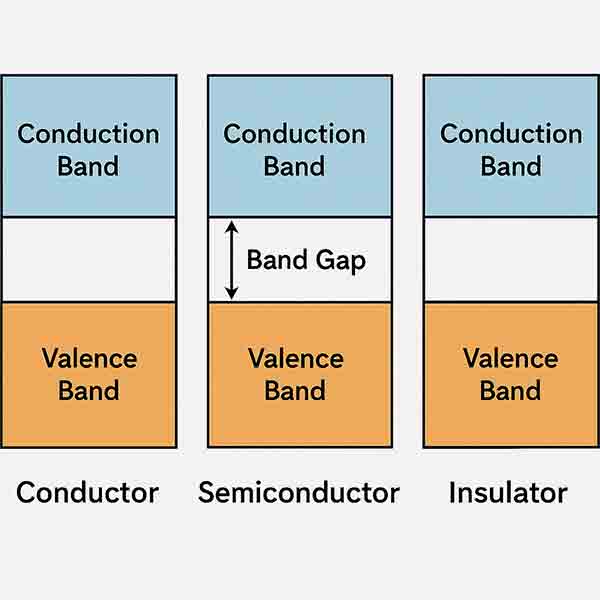
What is Band GaP?
Semiconductor band gap is the minimum amount of energy that is required to excite electron to free them from their bound state into a free state to conduct electricity. And is differnce between materials free state and its bound state and between the valence band and conduction band.
Below is a list of materials and their band gap. Let us know if you have any questions.
| Material | Band Gap (eV) |
|---|---|
| Silicon (Si) | 1.12 |
| Gallium Arsenide (GaAs) | 1.42 |
| Gallium Antimonide (GaSb) | 0.67 |
| Silicon Carbide (SiC) | 3.26 |
| Fused Silica | 9.0 |
| Single Crystal Quartz | 8.9 |
| Germanium (Ge) | 0.66 |
How do you calculate the energy gap of a material?
The energy gap (also called the band gap) of a material refers to the energy difference between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO). In a solid material, these are often referred to as the valence band and conduction band, respectively. The energy gap is a crucial property in semiconductors, insulators, and metals.
There are several methods to measure or calculate the energy gap of a material, depending on the type of material and the precise nature of the energy gap. These methods involve both experimental techniques and theoretical calculations:
-
Experimental Methods: Experimental methods are often used to determine the energy gap of a material.
-
Absorption Spectroscopy: The energy gap can often be determined from absorption spectroscopy, where the material absorbs light (or other electromagnetic radiation) and transitions from the valence band to the conduction band occur. By finding the point at which absorption begins to increase rapidly (the absorption edge), one can determine the band gap.
-
Photoluminescence Spectroscopy: In this method, a material is excited with a laser or other light source, and the emitted light is analyzed. The peak energy of the emitted light can be related to the energy gap of the material.
-
Electron Energy Loss Spectroscopy (EELS): This is a technique used in electron microscopes. A beam of electrons is sent through a thin sample, and the energy lost by the electrons due to interactions with the sample is measured. The energy loss can be used to determine the band gap.
-
-
Computational Methods: Theoretical methods can be used to calculate the energy gap. The computational approach involves solving the Schrödinger equation for the material's electrons, which is typically done using methods like density functional theory (DFT), GW approximation, or time-dependent DFT. These methods can give very accurate results, but they are computationally intensive and require significant expertise to carry out.
Keep in mind that all these methods have their strengths and weaknesses, and the most appropriate one will depend on the specific circumstances, including the type of material and the resources available. For complex materials or situations, multiple methods may be used in combination to get a more accurate determination of the energy gap.
FAQs About Bandgap in Semiconductors
- What is a bandgap in semiconductors?
- The bandgap is the energy difference between the valence band and the conduction band in a semiconductor. It determines whether a material conducts electricity.
- Which materials have a wide bandgap?
- Materials like silicon carbide (SiC), fused silica, and quartz have wide bandgaps. They are used in high-power and high-temperature applications.
- Why is bandgap important in solar cells?
- The bandgap determines the range of light wavelengths a material can absorb. Materials with smaller bandgaps can convert more solar energy into electricity but may overheat.
Low Band Gap Semiconductor Which Can Be Used to Fabricate Solar or Photovoltaic Cells
A Graduate Research Assistant requested help with the following.
Can you please let me know if you have low band gap semiconductor which can be used to fabricate solar or photo-voltaic cell.
Also is there any option for us to customize the doping profile in the material.
I can provide a research paper which we would like to follow to fabricate InAs photodiode. The doping profile discussed in the paper is as below:
The InAs wafer studied in this work was grown by metalorganic vapour phase epitaxy on a 2” p-type InAs substrate. The wafer structure consisted of a 2 μm p+ layer (doped with Zn at a nominal concentration of 1 Å~ 1018 cm−3) followed by an 8 μm intrinsic layer, and finally a 2 μm n+ layer (doped with Si at nominal concentration of 1 Å~ 1018 cm−3), as shown in Fig. 1(a).
Here are the band-gaps of common semiconductor materials
- Si 1.11 eV
- Ge 0.67 eV
- InAs 0.354 eV
- InSb 0.17 eV
I presume that you are interested in InAs and InSb.
Both of those materials are available in wafers 2"Ø and 3"Ø.
Both materials are available as undoped n-type, doped n-type and doped p-type.
Wafers of both of these materials are available in (100) and (111) crystallographic orientations.
We can also produce by MOCVD Epi layers of above and other such semiconductors. Within the Epi layers we can customize the doping profile, the band-gap and other parameters.
We shall to get an offer for the following:
Item Qty. Description
GG04. 1/10 Epi wafers, 2"Ø×500±25µm, EJ Flats (two),
Substrate: p-type InAs:Zn[100]±0.5°, Nc=(1E18-3E18)/cc, EPD<10,000/cm²,
One-side-polished, back-side Alkaline etched
Epi Layer 1: 2.0µm thick, p-type InAs:Zn, Nc=1E18/cc,
Epi Layer 2: 8.0µm thick, undoped InAs:-, Nc<1E16/cc,
Epi Layer 3: 2.0µm thick, n-type InAs:Si, Nc=1E18/cc,
The MOCVD facility that would do such wafers, will run tests next week to verify if they can undertake it and what would be the cost. We do have the required InAs substrates in stock.
For your part, please verify if you can use thinner Epi layers and if you really need the p-type InAs:Zn layer if the substrate is already p-type InAs:Zn.
Reference #228706 for specs and pricing.
What Are the Effects of a Smaller Band Gap Than a Silicon Band Gap?
Having a narrower band gap in semiconductors makes them more conductive than other types. This is because thermal excitation creates a small population of the valence and conduction bands in a semiconductor. An applied electric field then rearranges this population to produce an electrical current. This effect is not consistent for all semiconductors. This is because the amount of energy required to promote electrons from a partially filled lower energy band to a completely empty, higher energy band is variable.
In regular semiconductors, the band gap is constant. This property is due to the presence of continuous energy states. However, in quantum dots, the band gap is size dependent. This effect is known as the quantum confinement effect and is a result of the fact that the semiconductor's size affects the band gaps. The larger the band gap, the less efficient the solar cell will be.
There are two ways to measure the bandgap energy of a material. First, you can calculate the bandgap of a material by using an electrical measurement method. Another way is to use spectroscopy. You can calculate the energy in a semiconductor by using an optical ellipsometry. The slope of the lnR vs. 1/T graph can be used to calculate the energy in a semiconductor.
Another way to determine the energy required for electrons to jump between two bands is by comparing the energy of the valence electrons of one material with the energy of its valence electrons in another. In addition to increasing the bandgap, you can also measure the atomic radius of germanium and silicon. The latter is a semiconductor with a large band gap, which is called a wide-band-gap semiconductor.
When it comes to the energy level, the bandgap energy is measured by the k vector. The k vector indicates the energy of the highest valence state. In contrast, the bandgap of the lowest valence state is the same. Therefore, these two measurements are not related. When these two variables are compared, the k vector will determine the energy in the valence and conduction band.
A smaller bandgap has many advantages over a silicon-based semiconductor. The bandgap is an important property of semiconductors that determines their ability to function under different conditions. Its larger bandgap is beneficial in applications that require fast switching and higher power, and it will also be more efficient in absorbing broad spectrum sunlight. It is essential to choose the right bandgap for semiconductors when the temperature is higher than 400 degrees.
When the temperature is higher than the bandgap, the valence electrons of silicon will be more energetic. Hence, the energy of the semiconductors decreases. As a result, the energy of photons is wasted as heat. Thus, the energy of the semiconductor is wasted. Besides, a smaller bandgap increases the energy of a solar cell.
While Si has a smaller bandgap than a silicon, GaAs and SiC semiconductors have a larger one. A narrower bandgap increases the temperature of a material. When the temperature is higher than the bandgap, the semiconductor is not conductive. A wider bandgap can prevent a malfunction. That means a device can function at a lower temperature.
When a material is in a solid state, it has a bandgap. In semiconductors, a smaller bandgap decreases its electrical conductivity. It is important to remember that a wider-bandgap means higher energy. So, it is best to avoid materials with a narrower-bandgap. It is necessary to consider all the possible side effects of the bandgap before deciding on a material.
