LPCVD Si3N4 Stoichiometric Nitride
A scientist the following small quantity quote for their research.![]()
Could you please supply us with a quote for the x5 wafers (realise minimum order quantity may be 25 but need to ask) of the following:
- Diameter: 150+/-0.2 mm
- Material: Si
- Grade: Prime
- Growth method: CZ
- Ori: <100>+/- 0.5o
- Resistivity: 10-20 ohm-cm (but does not matter really)
- Thickness: 675+/- 25um
- Front surface: Polished
- Back Surface: Acid Etched
- Water with notch
- LPCVD Si3N4: Stoichiometric Nitride 500 nm. Greater than 500 nm also ok.
Find out what we quoted.
Get Your Quote FAST! Or, Buy Online and Start Researching Today!
University Wafer offers a comprehensive selection of Nitride on Silicon (SiN) wafers suitable for various research and industrial applications. These wafers are available in multiple diameters, including 50.8mm, 76.2mm, 100mm, and 150mm, with options for different film types and stress levels.University Wafer+3Silicon Wafers in Stock+3University Wafer+3
Key Product Options
-
Deposition Methods:
-
LPCVD (Low-Pressure Chemical Vapor Deposition): Provides stoichiometric films with high thermal stability.
-
PECVD (Plasma-Enhanced Chemical Vapor Deposition): Offers low-temperature deposition suitable for wafers requiring minimal thermal processing. University Wafer+2University Wafer+2University Wafer+2
-
-
Stress Levels:
-
Stoichiometric: Standard high-stress films.
-
Low Stress: Approximately 250 MPa tensile stress.
-
Super Low Stress: Less than 100 MPa tensile stress, allowing for thicker nitride layers up to 4 microns without cracking. University Wafer+10University Wafer+10University Wafer+10University Wafer+5University Wafer+5University Wafer+5University Wafer+1University Wafer+1
-
Applications
These Nitride on Silicon wafers are utilized in various fields, including:
-
Telecommunications: For optical communication systems involving wavelength division multiplexing and signal routing.
-
Quantum Photonics: Supporting on-chip photon generation and manipulation.
-
Sensing: Used in biosensing and environmental sensing due to chemical inertness.
-
LIDAR Systems: Integrated into systems for autonomous vehicles and mapping.
-
Frequency Combs: Essential for precision spectroscopy and metrology.
-
Data Centers: Enhancing optical interconnects for improved data transfer speeds. University Wafer
For detailed specifications and to explore the full range of available products, you can visit University Wafer's Nitride on Silicon category page: Silicon Wafers in Stock.
If you have specific requirements or need assistance selecting the appropriate wafer for your application, feel free to provide more details, and I can help guide you further.
What Is LPCVD?
LPCVD, Nitride or Liquid Phase Chemical Vapour Deposition (CVD), is a process that forms layers of polysilicon on a substrate. The process is performed in a vacuum and involves the use of plasma to deposit the layer. LPCVD films can contain many different types of materials, and have excellent conformal step coverage. Common LPCVD film compositions include polysilicon for gate contacts, thick oxides for global planarization, and nitrides and dielectrics.
The first question that you may ask is what is LPCVD? LPCVD stands for Low Pressure Chemical Vapor Deposition. LPCVD is a process where an atomic layer of material is deposited using pressure and a vacuum. A deposition temperature of approximately 425 to 900 degC is used to deposit a single layer of semiconductor material. Silicon dioxide can be deposited at temperatures of 650 degrees Celsius.
The second question that you might have is what is LPCVD? LPCVD is a chemical deposition process that is popular in the electronics industry. This type of process is a method of laying down a thin layer of material using a reactive gas. The gases in the system include oxygen, sulfur, and hydrogen. The process uses extremely low pressures and can be used to deposit polysilicon and silicon nitride.
Another question that may come to your mind is what is LPCVD. LPCVD is a form of sputtering. It is a process that uses a molten gas to deposit a thin film of material. The gases are heated above the ambient temperature of the gas source and then evaporated, leaving behind a thin film of sputtering film. Then, the thin film is deposited onto a surface.
LPCVD is a popular method for creating crystalline materials. It uses an oxygen-based plasma to deposit SiO2 on a surface. The deposition process is low-temperature and high-deposition rates. Unlike thermally grown oxides, LPCVD uses an organic gas, silane. These two are not the same, but both are a form of silicone. They are important for electronics.
While thermal growth of oxides is the primary method for producing crystalline materials, LPCVD produces films that have high residual stresses and gradients of stress throughout the film. Unlike thermal growth, LPCVD deposits materials at a low temperature. Its benefits are numerous and its uses are nearly limitless. Its applications are countless. With its benefits and low-temperature process, it is ideal for making MEMS devices.
LPCVD is a process that produces high-density, low-temperature, and deposited oxides. The process can be used to create both metal and semiconductor materials. However, a downside of LPCVD is the risk of bacterial contamination. In addition to being a health risk, LPCVD can damage electronic components. The risk of infection can be reduced to minimal, even zero.
LPCVD produces films with high residual stresses and a high degree of gradient stress throughout the film. This can be detrimental to MEMS devices. Fortunately, LPCVD is a viable alternative to conventional crystalline deposition processes. Besides its low cost, LPCVD produces high-quality films that are characterized by low residual stresses and high-temperature processes. The film thickness and the process time are the most important aspects of LPCVD.
LPCVD produces films with high residual stresses that are important for the performance of MEMS devices. It can also be used to make films that are resistant to heat. In addition to film-deposition, LPCVD can also be used for the formation of thin-films. The deposition rate is much higher than thermally grown oxides. Unlike the latter, LPCVD is a high-rate process.
Unlike conventional CVD, LPCVD uses heat to deposit materials. The lower pressure used in the process decreases unwanted gas phase reactions and enhances uniformity across a substrate. Similarly, LPCVD produces good conformability and high-quality films. The resulting layers are thin and uniform in thickness, with excellent adhesion and reactivity. If a process is successful, it can produce nanoscale polymer materials.
LPCVD is an efficient method of depositing silicon. It uses plasma and a vacuum to turn silicon carbide powder into vapor. Using a gaseous plasma, it deposits a thin film on a substrate. This process is used for silicon, nickel, and other semiconductor materials. A wide range of metals can be deposited. A high-grade insulator made from copper is more expensive.
What Is LPCVD Used For?
LPCVD is a process used to grow polysilicon. This type of deposition is very versatile and requires only pure silane as a feedstock. The films produced by LPCVD have high residual stress and have gradients throughout their thickness. Because of these properties, LPCVD films are not ideal for MEMS devices, but they are often very effective in other applications. Here are a few examples of LPCVD film applications.
LPCVD is the best way to grow silicon dioxide. The process is fast and allows for low temperatures. Typical 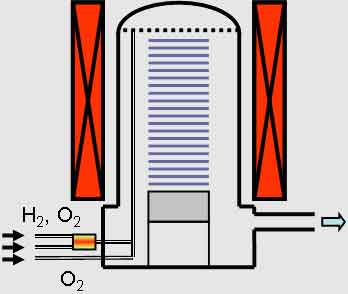 applications include trench filling, coating higher aspect ratio features, and electrical isolation. The process requires vacuum-tight process tubes, which must be water-cooled. The Tystar Corporation has developed recipes for nano materials CVD for over 30 years with national labs and universities. Both the APCVD and LPCVD methods use a Tystar furnace.
applications include trench filling, coating higher aspect ratio features, and electrical isolation. The process requires vacuum-tight process tubes, which must be water-cooled. The Tystar Corporation has developed recipes for nano materials CVD for over 30 years with national labs and universities. Both the APCVD and LPCVD methods use a Tystar furnace.
This process is useful for a wide range of applications. It produces silicon oxide, a semiconductor material with excellent dielectric and mechanical properties. Silicon oxide is often used in memory devices, MEMS, and other devices. The high-quality film made by LPCVD is suitable for thermally grown films. There are a variety of materials that can be produced using LPCVD. If you have a specific application in mind, it is important to choose the right material for your process.
LPCVD processes can produce polysilicon, silicon nitride, and silicon dioxide. Most LPCVD films are somewhat conformal and can offer sidewall protection for structures. Depending on the type of LPCVD process, the sidewall coverage can be quite extensive or very small. The higher the temperature, the greater the conformality, though the risk of keyhole formation remains.
LPCVD can produce silicon dioxide in various forms. The first step is to mix the precursor gas with silicon nitride. This gas will react with the substrate and generate a thin film of silicon oxide. The resultant film is a thick film with a low dielectric constant. The best quality LPCVD is the silicon oxide formed by reaction. This process is very suitable for coating in the earliest stages of IC manufacturing.
LPCVD is used to deposit silicon on substrates. It can be applied in the fabrication of various semiconductor devices. This process is a good alternative for a variety of IC applications. The process has many advantages. Typically, LPCVD films are more uniform, have less defects, and have a higher step coverage than thermally grown films. This technique is a good option for a wide range of semiconductor products.
LPCVD films can be tuned to specific properties. The resulting films are more uniform and have higher breakdown voltages. In addition, LPCVD films can be tuned to meet specific requirements. For example, higher process temperatures are used for LPCVD silicon oxide. While a lower temperature is better for thermal oxidation, LTO has less conformality. The same holds true for LPCVD-based oxidation.
LPCVD is an excellent solution for forming thin films of silicon dioxide. The ammonia generated during the reaction is more conformal than the deposited silicon dioxide. The ammonia used is the best choice for silicon oxide. LTO also has excellent gap filling capabilities and can be used for a wide range of IC applications. So, LPCVD is an excellent solution for your IC needs.
LPCVD processes are best suited for applications involving high-quality silicon. For example, silicon nitride has an exceptionally high density and is the most conformal LPCVD process. It is also very effective for trench filling and coating with higher aspect ratio features. In contrast, thermal oxide can be adapted to be used in the same way as low temperature oxide. This method can be a good alternative for making anti-reflective films, but it has less adherence and a higher melting point than LTO.
LPCVD processes can be used for a number of applications. The most common is epitaxial silicon deposition. This process is more expensive than other methods and can only process a small number of wafers. It is also limited in wafer processing capacities, so it's important to have a large amount of spare capacity. When LPCVD is applied to a large volume, it is important to consider the quality of the materials.
What is the Difference Between LPCVD and PECVD?
There is an important difference between the two processes. Both techniques generate volatile species, which can influence the deposition process. In LPCVD, silicon nitride is used as a stressor and etch stop, while PECVD produces silicon oxide. In PECVD, the hydrogen content determines the residual film stress. Higher pressure and temperature lower the hydrogen content. Plasma conditions also influence the amount of volatile species.
LPCVD starts with islands on the surface of the substrate and merges to form a continuous film. There is no need to use a silicon substrate in this process, since the underlying metal layer does not require a silicon substrate. In PECVD, the process is conducted at the highest possible temperature, typically between 10 and 1,000 Pa. The standard room pressure is 101,325 Pa. Moreover, the deposition temperature is much lower. This feature is important in many applications, because a thinner film will not affect the performance of the device.
The deposition temperature of PECVD is much higher than that of LPCVD. In LPCVD, the reactants are introduced in islands to form a thin film. When a thin film is deposited, this layer combines with the next layer. The result is a film with almost no hydrogen content. In contrast, LPCVD films have a high hydrogen content and pinholes. But, they have a higher deposition rate and a longer film life than LPCVD films.
LPCVD is a process in which the reactant gases are introduced between parallel electrodes. This process involves using ozone to catalyze reactions on the surface of the substrate. Compared to LPCVD, APCVD has a higher deposition rate and more flexibility. If you're interested in using a silicon substrate, PECVD will be the way to go.
LPCVD starts as islands on a silicon substrate. Then, they merge together to form a continuous film. Unlike PECVD, LPCVD uses the highest temperatures to deposit films. For example, a silicon substrate is not needed for a silicon nitride film. The resulting film thickness will depend on the temperature of the film. The higher the temperature, the thicker the film.
The LPCVD process involves introducing the reactant gases between parallel electrodes. The gases are then excitably coupled with the plasma to produce a reaction. The product of the reaction is deposited onto a substrate. The temperature of this deposition process is low, between 250 and 350 degC. While the temperature required for CVD is approximately six hundred and eighty degrees Celsius, the lower temperatures make the process more economical.
In PECVD, reactant gases are introduced between parallel electrodes. The reaction produces a plasma that induces a chemical reaction. The reaction product is then deposited on the substrate. During the process, the substrate is heated to 350 degrees. Unlike in ALD, the PECVD film is less flexible than its LPCVD counterpart. In addition, it has a higher deposition rate.
LPCVD has a higher temperature than PECVD. It uses a plasma to provide energy to the reactants. While PECVD uses a high temperature, it is a semi-clean method for producing silicon-based materials. When LPCVD is used, a silicon substrate is not necessary. The two processes differ in the types of film they use. The primary difference between LPCVD and PECVDS is the temperature and the deposition rate.
LPCVD is more common than PECVD. The two processes are similar but there are several important differences. One is that LPCVD uses a silicon substrate and PECVD utilizes a tungsten-based substrate. In LPCVD, the temperature range is lower than that of PECVD. The temperature is important for some applications, but it is also important for safety.
While LPCVD can deposit low-k dielectrics, it requires higher-temperature and pressure. The gas flow rate and chamber pressure are optimized for good wafer uniformity and good oxidation. LPCVD equipment can reach a temperature of about 350-400degC. Increasing the DCS/NH 3 ratio decreases the deposition rate. The difference between LPCVD and PECVDE is the difference in deposition rate.
LPCVD Applications
LPCVD is a technique for chemically vapor deposition of thin films. The process uses an ion source and a current that flows through a coil. The current traps ions and electrons close to the surface of the coil, resulting in a plasma that is radially nonuniform. The plasma's intensity is greater near the coil's surface than on its inner surface, which leads to a reduced concentration of reactive species and ion bombardment on the tube's interior.
High temperatures and high concentrations of oxygen or nitrogen are necessary for LPCVD to work. This 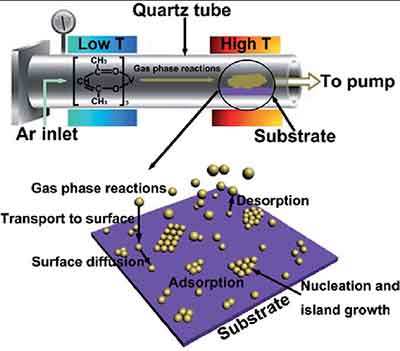 increases the amount of ion bombardment, which may result in a nonuniform film removal. The process is more efficient when it is able to reach temperatures in the low teens. However, the high temperatures are not suitable for production-scale LPCVD systems. Because the film's thickness can be controlled, there are many limitations associated with LPCVD.
increases the amount of ion bombardment, which may result in a nonuniform film removal. The process is more efficient when it is able to reach temperatures in the low teens. However, the high temperatures are not suitable for production-scale LPCVD systems. Because the film's thickness can be controlled, there are many limitations associated with LPCVD.
Prior LPCVD patents describe a process that involves in-situ cleaning of the LPCVD tube. Several of these techniques involve the use of electrodes on the outside of the tube. The electrodes are either longitudinal along the length of the tube or circumferentially. Some of these techniques can be used on a variety of substrates, including LPCVD tubes and RPE bell jars.
The LPCVD process requires higher temperatures than other methods of chemical vapor deposition. This limits the materials that can be applied to the samples. Further, the species lifetime is less than the time needed for them to travel from the plasma to the deposits at the far end of the LPCVD tube. Thus, the material etching process is more effective at higher temperatures. The higher temperatures of the LPCVD tube also reduces the amount of contaminants in the sample.
LPCVD is a method for chemically vapor deposition of nanostructured materials. Its ion-based nature allows it to be used for a variety of applications. One example is in the field of biomedical devices, such as biosensors and cell phone sensors. This technology is also useful for the development of high-quality polymers. Aside from advancing the field of medicine, LPCVD has a wide range of applications.
The LPCVD method uses higher temperatures than other deposition processes. This means that it is limited to certain materials on the sample. Nonetheless, this is an effective method for manufacturing conductive materials, and is a popular way to make high-quality semiconductor devices. This technique also allows for more flexibility with the type of materials on the sample. A high-quality LPCVD process has many potential benefits for industry and research.
LPCVD films can be tuned for specific properties. The thermal oxidation process can be adjusted to produce high-quality, uniform films that exhibit higher breakdown voltages. The lower-temperature process can produce high-quality, low-stress films. Another benefit of LPCVD is the ability to modify the temperature. Changing the temperatures of the LPCVD process can change the characteristics of the material.
The LPCVD method can be used in a wide variety of applications. It can be used to create complex nanostructures and other materials. Several prior patents describe in-situ cleaning of LPCVD tubes. During this process, an inductive coil is placed inside a quartz tube. The RF power causes a plasma to form inside the LPCVD tube. Although this technique produces thinner films, the inductive coil produces a higher-quality plasma than the conventional methods.
LPCVD films can be tuned for specific properties. The process temperatures are typically selected to optimize film quality and yield. Increasing the temperature of the LPCVD process can also optimize film properties. Various LPCVD processes can be optimized for different purposes. For example, a TEOS film can be molded into a silicon wafer. The process can be customized for various substrates and can be calibrated to achieve desired thicknesses.
An LPCVD film is amorphous. Its properties do not vary significantly along the length of a tube or temperature gradient. Consequently, it can be used for various types of semiconductor devices. There are many advantages to this method. Unlike the cold-wall method, it is highly versatile and inexpensive. Its high volume makes it an excellent choice for solar cells and solar panels. The process also makes a highly transparent film.
What is the Difference Between MOCVD and CVD?
CVD and MOCVD are two processes that can deposit metals. They are similar in that they are both used for the deposition of copper and aluminum. The main difference between the two processes is that MOCVD can be used for the deposition of both copper and aluminium. It is used to deposit copper from organoaluminium and triisobutylaluminium. A CO2 or oxygen reaction occurs during the process, forming metal oxides or carbon dioxide. The difference between these two processes can be seen in their use in the fabrication of quantum well lasers and other components.
In order to answer the question, let's look at the differences between these two processes.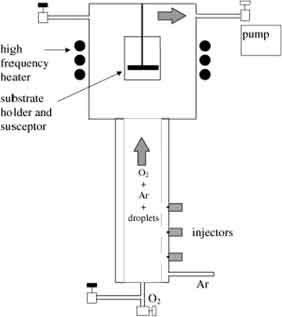 The first one is the most difficult to implement in small labs, while the latter is suitable for large-scale industrial production. The other one is easier to use and can be applied in research laboratories. It is a great choice for high-volume industrial manufacturing and large-scale manufacturing. ACVD is a popular choice for the production of thin films for the semiconductor industry.
The first one is the most difficult to implement in small labs, while the latter is suitable for large-scale industrial production. The other one is easier to use and can be applied in research laboratories. It is a great choice for high-volume industrial manufacturing and large-scale manufacturing. ACVD is a popular choice for the production of thin films for the semiconductor industry.
When comparing the two processes, MOCVD is generally more efficient for the fabrication of thin films and structures. In addition, it allows for fine-tuning, abrupt interfaces, and good dopant control. However, it is not suitable for making high-performance, power-hungry devices. The main difference between CVD and MOCVD is that MOCVD uses liquid precursors.
MOCVD and CVD are both chemical deposition processes. However, the former is the better choice for research labs and high-volume production. Both processes have their advantages and disadvantages. As the latter is more expensive, it is not practical to apply MOCVD in a lab setting. In addition, it is not suitable for small-scale productions. If you're in a hurry, it's better to use MOCVD.
As with CVD, MOCVD has the advantage of using lower temperatures for the deposition of metals. Both processes have their benefits and drawbacks. While CVD has a lower cost, MOCVD is more productive and is more efficient for low-volume manufacturing. In addition, it is easier to use than PVD. In the lab, you can use either one in the same process, but there are major differences between the two.
While there are similarities between MOCVD and CVD, MOCVD is the more advanced method. The vaporization of organic gases produces III-V semiconductors. This type of material is similar to LPCVD. In fact, it is easier to use in laboratory settings than LPCVD. In fact, it's not possible to do both processes with the same process. If you're not familiar with them, the difference between the two processes is not very noticeable.
Another technique that is similar to CVD is MOCVD. Both techniques use a liquid to deposit a material. The vaporization process is faster than the other, but it has a lower rate of growth. The difference between these two methods is important in order to avoid errors. In addition, MOCVD is much less expensive. It's difficult to run in small laboratories. The main downside of MOCVD is its high costs.
MOCVD is an advanced technique that enables the deposition of crystalline compound semiconductor thin films and structures. Compared to CVD, MOCVD offers high-precision control, and can be used for manufacturing of a variety of products. Both processes are best suited for industrial applications that require high-quality materials and require a low-temperature reactor. If you are working on a project that requires metal-based technology, MOCVD is the way to go.
MOCVD is a more complex method. It can achieve extremely high-purity crystalline compounds, allowing for abrupt interfaces and fine-tuning. In contrast, a CVD process requires higher temperatures, which can be expensive for smaller laboratories. In comparison to MOCVD, the latter is more widely used. It has more advantages than the former, including the ability to create crystalline structures.
