What is a Metalloid?
Which Element is a Metalloid?
Metalloids are elements that have properties intermediate between metals and nonmetals. Some common examples of metalloids include:
- Boron (B)
- Silicon (Si)
- Germanium (Ge)
- Arsenic (As)
- Antimony (Sb)
- Tellurium (Te)
- Polonium (Po)
These elements typically exhibit a mix of metallic and nonmetallic properties and are often found in a zigzag line on the periodic table between the metals and nonmetals.
Get Your Quartz Quote FAST! Or, Buy Online and Save!
Is Germanium (Ge) Non-Metal While it is Metal or a Metalloid?
-
Position in the Periodic Table: Germanium is located in group 14 of the periodic table, between silicon
 (a nonmetal) and tin (a metal). This positioning can cause confusion because it shares some characteristics with both neighboring elements.
(a nonmetal) and tin (a metal). This positioning can cause confusion because it shares some characteristics with both neighboring elements. -
Physical Properties: Germanium exhibits metallic luster and is a good conductor of electricity, especially at higher temperatures, which are typical properties of metals. However, it also has some properties of nonmetals, such as being brittle rather than malleable.
-
Chemical Properties: Chemically, germanium forms covalent bonds, like nonmetals, and its oxide is more similar to those of nonmetals than metals.
-
Historical Context: When germanium was first discovered, the criteria for classifying elements were less well-defined than they are today. As a result, early classifications might have varied, contributing to lingering confusion.
-
Variability in Definitions: Different sources or educational systems might emphasize different criteria for classifying elements, leading to variations in how germanium is categorized. Some sources might place more emphasis on its metallic properties, while others might highlight its nonmetallic characteristics.
Overall, germanium is best classified as a metalloid due to its intermediate properties between metals and nonmetals.
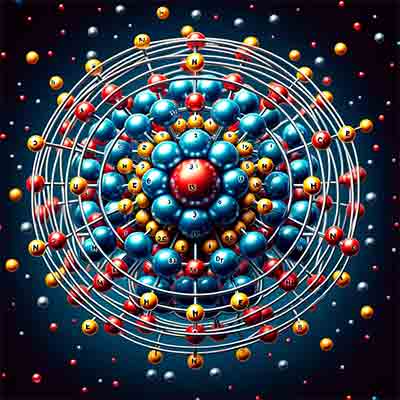
The image above depicts the basic structure of germanium's atomic arrangement. Here are the key points to verify its accuracy:
-
Protons and Neutrons in the Nucleus: The nucleus should contain 32 protons, and the number of neutrons typically for germanium-74 (the most common isotope) is around 42. The illustration should reflect a roughly accurate number of neutrons alongside the protons in the nucleus.
-
Electron Configuration: Germanium has the following electron configuration:
- 1st shell: 2 electrons
- 2nd shell: 8 electrons
- 3rd shell: 18 electrons
- 4th shell: 4 electrons
-
Representation of Particles:
- Protons as red spheres.
- Neutrons as blue spheres.
- Electrons as yellow dots in distinct energy levels.
Given these points, the image should be cross-checked for these specific details to confirm its accuracy. The illustration should align with the standard scientific representation of germanium's atomic structure.
