I want to use a silicon wafer for XRD measurements of polymer coatings. I am not sure which type of silicon wafer is suitable for this. Could you please give me feedback about this?
UniversityWafer, Inc. – Precision Wafers for Accurate X-Ray Diffraction (XRD) Results
Achieve accurate and consistent results in your X-Ray Diffraction (XRD) research with UniversityWafer, Inc.'s high-quality substrates. Our wafers feature exceptional surface uniformity, ensuring reliable and precise diffraction readings. Accurate wafer orientation further enhances the quality and reproducibility of your experimental data, benefiting researchers in materials science, semiconductor technology, nanotechnology, and crystallography.
(XRD) research with UniversityWafer, Inc.'s high-quality substrates. Our wafers feature exceptional surface uniformity, ensuring reliable and precise diffraction readings. Accurate wafer orientation further enhances the quality and reproducibility of your experimental data, benefiting researchers in materials science, semiconductor technology, nanotechnology, and crystallography.
🔬 Why Researchers Choose UniversityWafer, Inc.:
-
✅ Uniform wafer surfaces for clear, accurate diffraction patterns
-
✅ Precise wafer orientation enabling reproducible results
-
✅ Superior quality substrates saving valuable research time and resources
-
✅ Technical expertise for custom solutions and reliable support
📚 Ideal for applications including:
-
Semiconductor and electronic materials research
-
Advanced crystallographic analysis
-
Thin-film and nanostructure studies
Silicon Wafers for X-Ray Diffraction
A scientist requested the following quote:
UniversityWafer, Inc. Quoted:
Si Item #8215
32mm P/B(100-9 deg towards (001) 2,000+/-50um SSP > 10 ohm-cm
Zero X-Ray Diffraction Plate with Central Sample Pocked 5mm0x200um, NO FLATS (Contact for Pricing)
[Q] This price is only for one wafer?
[A] Yes, it is, but item #8215 is a special wafer that guarantees Zero X-Ray Diffraction.
[Q] And may I use it also for AFM?
[A] Yes, you can.
[Q] If it is only one wafer, do you have cheaper options?
[A] We have many, but only #8215 is Zero X-Ray Diffraction.
Contact us for all wafer options.
Get Your Quote FAST! Or, Buy Online and Start Researching today!
510 Oriented Silicon Wafer For Diffraction Test
A Materials Characterisation Technician requested a quote for the followiong.
I am looking to buy some Silicon wafer with the (510) orientation. This will be used for zero background X-ray powder diffraction test.
I am just wondering if UniversityWafers offers cutting to size service upon the purchase of a wafer disc (let's say a 100 mm one)? The goal is to cut 25 mm discs out of the bigger disc (as many pieces as physically possible). Please advise if this is possible.
Reference #325700 for specs and pricing.
What Wafers are Used for Synchrotron XRD Measurements?
Scientist asked for the following quote:
Please send me a quote for "100um+/-10um 6" thin Silicon, SSP, $ each, minimum order of 5 wafers" for synchrotron XRD measurements. We require wafers with a thickness range between 100 microns to 200 microns. Do you have Si wafers with the above thickness.
UniversityWafer, Inc. Quoted.
Si Wafer
6", 100+/-10um, SSP
Quantity: 5 Wafers
FOB Price: $Reference #266721 for pricing.
Delivery Time: 2 Weeks
Thin Silicon for XRD Transmission Measurements
A posdoc in a Nanostructured Electrodes for Biocatalysis lab requested the following quote:
I would like to order some Wafers and check the availability. I have a quiete recent list of available wafers from you. However, before ordering, I would like to have some offers for ultra thin Si wafers for XRD transmission measurements. I would be pleased about a quick reply, so that I can order as soon as possible.
Reference #111054 for specs and pricing.
Uniform Silicon Specs for Accurate XRD Readings.
What Substrates are Used for GI-XRD?
A PhD candidate requested the following quote:
I am looking for silicon that will have no diffraction peaks so that I can use it as a substrate when doing GI-XRD and get minimal signal from the substrate. I believe that {111} off cut silicon will do the trick. Do you have any prime grade silicon of the type that should work for my purposes? Any thoughts or suggestions?
UniversityWafer, Inc. Quoted:
| Typ/Dop | Ori. | Dia. | Thick(µm) | Pol | Res Ω∙cm | Comment |
|---|---|---|---|---|---|---|
| P/B | [510] | 2" | 1,550 | DSP | FZ 2,879–3,258 | Zero Diffraction Plate, NO Flats, Individual cst, Sold in packs of 6, 5, 5 wafers |
Reference #289995 for specs and pricing.

What is X-Ray Diffraction?
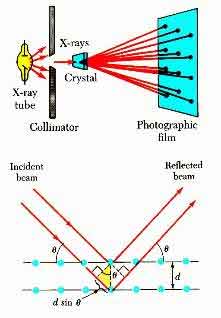
X-Ray Diffraction is an experimental technique that determines the atomic and molecular structure of a crystal. Specifically, it measures the diffraction of X-rays because the crystalline structure of the material causes the beam of incoming X-rays to diffract. Moreover, it helps scientists to understand how a crystal forms, including the role of electrons and protons.
The diffraction of an x-ray is based on the theory of eigenmodes, which states that a wave that has 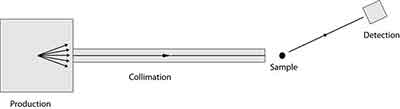 the same frequency as the material's atomic structure will cause a pattern of eigenmodes. In other words, an x-ray diffraction image is a spherical slice of reciprocal space. If the atoms in a crystal have a regular arrangement, the diffraction will result in the same length of kout and kin. The resulting diffraction image will therefore have the same values of q.
the same frequency as the material's atomic structure will cause a pattern of eigenmodes. In other words, an x-ray diffraction image is a spherical slice of reciprocal space. If the atoms in a crystal have a regular arrangement, the diffraction will result in the same length of kout and kin. The resulting diffraction image will therefore have the same values of q.
 X-Ray diffraction is a type of analysis using x-rays to determine the structure of matter. The method involves scattering X-rays and allowing them to pass through a variety of materials. It is especially useful for studying non-crystalline materials, as a diffraction image can provide information on the crystallinity, phase, and preferred orientation.
X-Ray diffraction is a type of analysis using x-rays to determine the structure of matter. The method involves scattering X-rays and allowing them to pass through a variety of materials. It is especially useful for studying non-crystalline materials, as a diffraction image can provide information on the crystallinity, phase, and preferred orientation.
When an electromagnetic wave impinges on a crystal, it bounces off atoms in a regular pattern. The angle th between the x-ray beam and the crystal is positive. When a beam is at a higher angle, it cancels out the previous one. This creates a new beam with a higher amplitude. The greater the intensity of peaks, the more distinct the spacing between atoms is.
X-Ray diffraction is a common type of scattering. The diffraction result is a plot of the intensity of the signal for various angles of diffraction. The two theta positions are the spacing between atoms or crystals. The diffraction result corresponds to a specific position and a set of peaks. The higher the peak, the more distinct the spacing between the atoms.
 X-Ray diffraction is a type of diffraction. The atoms in a crystal are the scatterers. The diffraction pattern is created by an electromagnetic wave impinging on a regular pattern of scatterers. X-rays are used as diffraction signals because their wavelength is close to the distance between the crystal planes. There are many different kinds of diffraction patterns.
X-Ray diffraction is a type of diffraction. The atoms in a crystal are the scatterers. The diffraction pattern is created by an electromagnetic wave impinging on a regular pattern of scatterers. X-rays are used as diffraction signals because their wavelength is close to the distance between the crystal planes. There are many different kinds of diffraction patterns.
X-Ray diffraction images are a spherical slice of a reciprocal space. During a diffraction experiment, a sample rotates around the diffraction angle. As the sample rotates, a peak in the intensity of the diffraction pattern is created. Smaller values of 2 th are overlapping, and larger diffraction peaks are separated. The peak location is the center of the diffraction peak.
X-Ray diffraction images are spherical slices of reciprocal space. The atoms in a structure change direction with respect to the angle of diffraction. The diffracted beams have similar wavelengths. Hence, if both the diffraction peaks have the same wavelength, they cancel each other out. The result is a new beam with a higher amplitude.
X-Ray diffraction is based on the constructive interference of monochromatic X-rays with a crystalline sample. Usually, a cathode ray tube generates X-rays. These are then filtered and collimated into monochromatic radiation. The resulting diffraction pattern consists of peaks in a sphere.
The X-ray diffraction pattern is a diffraction pattern of an atom's energy. It is a form of energy-free light and is a form of optical radiation. The diffraction pattern is a snapshot of the atom's structure. The intensity of the X-ray diffraction is determined by varying the wavelength of the X-ray beam.
The X-rays' intensity is measured and then compared to a reference object. In other words, diffraction is an analysis of the atomic structure of a material. Its main function is to identify the composition of an object. The diffraction pattern of a material is a reflection of its composition. For example, a crystalline structure can contain a wide range of atoms.
What is X-Ray Diffraction Analysis?
The purpose of X-Ray diffraction analysis is to study the crystalline structure of materials. Unlike other analytical techniques, X-ray diffraction works by impinging an electromagnetic wave onto a crystal with a regular arrangement of scatterers. The scattered atoms form peaks, or diffraction patterns. These peaks are the result of the diffraction of a specific wavelength.
X-Ray diffraction is a method for analyzing crystals, which involves bombarding the crystals with X-rays. The diffraction pattern is then plotted. The two peaks represent the intensity of atoms and molecules and are related to the atomic arrangements of the sample. The higher the peak intensity, the more molecules are in phase and have distinct spacing.
X-Ray diffraction is a process that measures the intensity of X-rays as they pass through a sample. The wavelength of the incident x-ray beam is used to determine the diffraction angle. The intensities of the peaks are then correlated with the number of crystals and atoms in the sample. Using this method, the atoms in a given sample have the same d-spacing, which means that they have the same amount of spacing or phase.
X-ray diffraction is a technique for determining the atomic structure of a sample. This technique is based on Bragg's law, which states that the wavelength of an X-ray has the same frequency as the distance between atomic planes. As the wavelength of an ion is higher, the intensity of the x-ray is greater, which gives the diffraction pattern a higher resolution.
What is X-Ray Diffraction and Why is it Important?
X-ray diffraction is a fundamental concept in science. It is a process of determining the exact shape of a crystal by measuring its diffraction pattern. The most important piece of information behind diffraction patterns is the wavelength of the incoming beam. The wavelength of an X-ray depends on its diffraction angle and lattice spacing.
X-ray diffraction is a form of X-ray analysis. It is used to determine the crystal structure of a solid, which allows researchers to make measurements with more accuracy. The diffraction pattern is used to study the properties of materials and identifying how they behave under high-energy radiation. It is a popular technique for analyzing crystalline samples.
The diffraction peak is calculated based on the intensity of the X-ray reflections from the sample. The diffraction peak profile is continuously recorded and the size of the unit cell is a key determinant. If the sample has a surface that is too rough, the diffraction pattern will be noisy. To reduce noise, smoothing it will help in the analysis. The diffraction peak profile is most effectively fit to eight or ten data points. Using the sharpest peak as the step size parameter, the diffraction peak is measured at 80% of the total height.
Most materials are not single crystals. They are polycrystalline aggregates of many tiny crystallites in all orientations. If you change the experimental angle, you will see all of the possible diffraction peaks. This is how we determine the structure of material. The diffraction pattern is a key part of science, and can reveal the structure of many different materials.
X-ray diffraction is a critical tool in science. It can be used to measure the size of particles in a variety of industries. Aspects of materials can be measured in terms of size, shape, and atomic number. In contrast, nanoparticles are amorphous, so the diffraction peak will differ from a crystal.
X-ray diffraction is a technique that uses X-rays to reveal the structure of atoms in a compound. Various applications of X-ray diffraction are found in chemistry. It is used to study the structure of organic and inorganic compounds. The principle of diffraction is described below. It is an essential tool for scientists and engineers in many fields.
X-ray diffraction is an important tool in characterization. It is a method for determining the purity of a sample. It is commonly used in forensic science and can be used in many applications. This technique has numerous advantages. It is a cost-effective tool for detecting impurities. It can also be used in the laboratory to measure sample size.
X-ray diffraction is a method used in science and engineering. It is a technique for analyzing a crystal. X-rays pass through a crystal and are reflected back in a pattern called an X-ray diffraction pattern. The diffraction image is then a map of atoms in the sample.
X-ray diffraction is a process that involves the use of a specialized X-ray diffraction tube. It uses a high-speed X-ray beam to pass through a sample. The diffraction pattern may contain noise or a smooth surface. Typically, an optimum diffraction spectrum will have 8 to 10 peaks.
Diffraction is a method of analysis that allows you to obtain the structure of a crystal. The data obtained is important in the study of a crystal. The diffraction of a crystalline material is crucial for determining the crystal structure and other details. It is an essential method for many research purposes. It is an effective means for analyzing complex materials and determining their diffraction angles.
X-ray diffraction is a method used to determine the crystal structure of materials. The technique is a non-destructive means of characterizing a material and determining their properties. Generally, it is used to analyze uniform materials. This type of diffraction is also used for comparing materials to see how they react with the X-ray.
