What Wafer Specs are Needed for Microfluidics Research?
SOI Used For a Microfluidics Mold
A PhD candidate requested help with a research project.
Reference #211268 for specs and pricing.
Test Grade Silicon Wafers to Build Molds
A postdoctoral researcher requested the following:
What 100mm silicon wafer spec should we use to build molds (using photolithography), that we then use to cast microfluidic devices?
Reference #46809 for specs and pricing.
Substrates to Fabricare Microfluidic Devices
The term "microfluidics" can be confusing, because it is used in a variety of ways.
Microfluidics is a technique used in experiments that require little or no sample to complete. The process uses fewer materials and requires less time than larger experiments, so the costs are much lower and the overall precision is higher. Because fewer samples are needed, it improves the accuracy of experiments and decreases the detection limit of a compound.
UniversityWafer, Inc. has the substrate to help with your microfluidic substrates. From glass to silicon we offer numerous wafers that you can buy online easily.
Get your Soda Lime Glass Wafer Quote FAST! Or, Buy Online and Start Researching Today!
Microfluidics Research Papers
Below are a small list of UniversityWafer's role in microfluidics research.
High-throughput gene expression analysis at the level of single proteins using a microfluidic turbidostat and automated cell tracking
"SU8 Photoresist were deposited on clean polished silicon wafers (University Wafer) using a spin coater. The wafers were then aligned to the mask and exposed using a mask."
Braess’s paradox and programmable behaviour in microfluidic networks
"A 4-inch silicon wafer (test grade, University Wafer, Boston, MA) was cleaned with acetone and isopropanol and dried with nitrogen gas."
Microfluidic technology for cellular analysis and molecular biotechnology
"The designed micro-patterns were then transferred to 3 nch silicon wafers (University Wafer) to form masters using a negative photoresist SU8 2025..."
Investigating blood flow and antibiotic dosing using traditional microfluidics and novel 3D printed devices
"...spin coated (Laurell Technologies, North Wales, PA) onto a 4” wafer (University Wafer, South Boston, MA) using the following program: 500 rpm for 15 seconds, 1000 rpm for 30 secon..."
Analysis of Flow-Based Microfluidic Gradient Generators for the Study of Bacterial Chemotaxis
"100 mm single sidepolished silicon wafers were purchased from University Wafer. Wafers were cleaned using a series of acetone, methanol, isopropanol, and deionized water rinses, ..."
An Integrated Array-based Microfluidic Device for Parallel Loop-Mediated Isothermal Amplification (LAMP)
Silicon wafers were used as a substrate in the photolithography process to define mold for the fabrication of the micro-wells. Different sizes of wafers are available, from 1 to 12 inches (2.5 to 30.5 cm). The 3-inch (7.6 cm) silicon wafer (University Wafer, MA, USA) was selected as it was suitable to fit the design of the device.
Micofluidic Chips to Study Bacteria
A Postdoc requested the following quote for our most popular item below.
Si Item#452 - 100mm P/B (100) 0-100 ohm-cm SSP 500um Test grade.
The researcher will use the wafer to manufacture microfluidic ship to study e. coli (non-pathogenic) bacteria.
Reference #261960 for more information.
PDMS Microfluidics
A PhD in Chemical Engineering requested the following quote:
What Substrates are Commonly used for Fabricating Mold on for the Application of PDMS Microfluidics.
We are looking for a silicon wafer (4") which we will use for fabricating mold on for the application of PDMS microfluidics.
Which one is the common for this application?
UniversityWafer, Inc. Repied:
For fabricating molds intended for casting Polydimethylsiloxane (PDMS) microfluidic devices, the most commonly used substrates are those that allow for high precision and compatibility with established microfabrication techniques. Here are the widely used substrates:
Silicon Wafers: Usage: Predominantly used due to their compatibility with a wide range of microfabrication techniques such as photolithography. Advantages: High precision, smooth surface finish, and dimensional stability.
Glass: Usage: Utilized for creating high-precision molds, especially when features with high aspect ratios are required. Advantages: Chemically stable, smooth surface finish, and can maintain fine details well.
Photopolymers:
SU-8:
- Usage: Widely used as a negative photoresist material in the lithographic process to create molds with high aspect ratios.
- Advantages: Can create fine features and high aspect ratio structures, relatively inexpensive.
3D Printed Materials:
Resins:
- Usage: Employed in high-resolution 3D printing technologies for rapid prototyping of microfluidic molds.
- Advantages: Allows for quick turnaround time in prototyping, with a reasonable level of detail.
In selecting a substrate for creating a master mold for PDMS microfluidic devices, it is critical to consider the necessary feature sizes and aspect ratios, the available fabrication technologies, and the required surface finish of the mold to ensure the production of high-quality PDMS microfluidic devices. Generally, silicon wafers and SU-8 photopolymers remain the popular choices due to their precision and compatibility with lithography-based fabrication processes.
Reference RFQ #224281 for specs and pricing.
75mm Silicon Wafers to Fabricate Microfluidic Devices
A graduate student requested the follownig quote:
I’m currently working on a budget for microfluidic device which uses 75mm silicon wafers. I’m not sure of what perimeters would be needed for those.
UniversityWafer's Engineer replied:
If you are doing micro-fluidic devices then presumably you etch in silicon wafers various channels, membranes, orifices, cantilevers, etc. The properties of the required wafers, depend on the etching process used.
Here are some parameters that you should consider and specify:
- Standard wafer diameter is 76.2±0.5mm - I you must have 75mm diameter then specify diameter and tolerance.
- Specify wafer thickness - 380±25µm is standard
- Specify Silicon dopant and resistivity - If you specify that the wafers be "undoped" that implies that they are of very high purity and resistivity (Resistivity is a measure of purity - the higher the resistivity, the purer the silicon, the higher the cost) - so do not do that. Generally, you should specify Resistivity as (1-100)Ohmcm. If your etching process depends on electro-chemical properties of the Silicon then you need to specify Boron doped Silicon (p-type) or Phosphorus doped Silicon (n-type). If your etching process is not sensitive to Silicon conductivity type then state "Doping immaterial" rather than "Undoped".
- If you use an etching process which which depends on p-type to n-type transition as the etch stop, then you may want to specify Silicon Epi wafers where the substrate is p-type and the Epi layer is n-type (or vice-versa). Epi layer can be anywhere form 5 to 150µm thick.
- If you use anisotropic etch (as is most common) then you need to specify Crystallographic orientation of the silicon wafer surface - (100) orientation is most commonly used, but you may need (110) orientation wafers for etching deep trenches with perpendicular walls.
- Generally Single-side polished wafers are adequate (but double-side-polished wafers are also available, if you intend to use both sides of the Silicon wafer). Surface roughness is normally about 1nm on rms basis. This is a result of the CMP polishing process used and is not measured so you should not attempt to specify it. Total Thickness Variation (TTV) relates to how well one can focus on the wafers and so how fine details you can etch. TTV<10µm is standard, TTV<5µm is extra quality, TTV<1µm is possible but very expensive. TTV relates to wafer thickness and presupposes that the wafer is used clamped to a flat reference surface (as is normal). If wafer is used "free-standing", so it rests on a flat reference surface touching it at just 3 points, then the Bow and Warp parameters are significant. For 3"Ø wafers Warp<30µm is standard (Bow is generally half of Warp although they are normally specified as the same, for example Bow/Warp<30µm), anything less incurs extra cost.
- Silicon is normally crystallized by the CZ process. This results in dissolved Oxygen content of about 20ppma. Some etch processes are sensitive to Oxygen content. In that case you may consider using FZ crystallized Silicon with dissolved Oxygen < 1ppma, but FZ Silicon is more expensive.
There are many other things that one can specify about Silicon wafers, but fhr MEMS and micro-fluidics above are probably most significant.
Reference # 221915 for specs and pricing.
Silicon Substrates Used to Study Extracellular Vesicles
A medical student requested help with the following:
Quantity and Specs
447 (76.2mm test grade)
452 (100mm test grade)
I'll be using the wafers for a number of microfabrication applications, mostly microfluidic devices to study extracellular vesicles.
100mm Silicon Wafers to Fabricate Master Molds
A biomedical PhD candidate requested a quote for the following:
I'm looking at 100 mm Si wafers (Prime grade, ID 809) for use in microfluidic master mold fabrication. It should be sent to you in the next couple of days.
I wish to purchase a number of Si wafers. I was wondering if your business accepts purchase orders?
Reference #M5G 1M1 for specs and pricing.
Substrates are Used to Fabricate Microfluidic Master Molds
Microfluidic master molds are generally fabricated using a variety of substrates to suit different applications and manufacturing techniques. Here, I outline some of the common substrates used:
-
Silicon: Traditionally, silicon wafers have been a popular choice for fabricating microfluidic master molds. Silicon wafers offer high precision and are compatible with established microfabrication techniques such as photolithography.
-
Glass: Glass is another common substrate used in the fabrication of microfluidic master molds. Similar to silicon, it offers high precision and is compatible with various microfabrication techniques.
-
Photopolymers:
- SU-8: A commonly used negative photoresist material, SU-8 is popular for its ability to create molds with high aspect ratios and fine features.
-
Polymethyl Methacrylate (PMMA): PMMA is often used due to its transparency and ease of machining, enabling the creation of molds with complex geometries.
-
Polydimethylsiloxane (PDMS): Although PDMS is more frequently used as a material to create microfluidic devices from a master mold, it can also be used to create master molds themselves, particularly in rapid prototyping and in educational settings due to its low cost and ease of use.
-
Metals: In some cases, metal molds can be used, created using techniques such as micromilling or electroforming. These can offer high durability and precision.
-
3D Printed Materials:
- Resins: With the advent of high-resolution 3D printing technologies, resins are becoming increasingly popular for rapid prototyping of microfluidic molds.
- Polylactic Acid (PLA): Another material commonly used in 3D printing, which can be used for creating molds, especially in early-stage prototyping.
-
Plastics: Various plastics can be used, especially in combination with CNC machining, to create molds with relatively large features or for rapid prototyping purposes.
-
Agar and Gelatin: In biological applications, agar and gelatin can be used to create temporary molds for microfluidic devices.
-
Ceramics: In some specialized applications, ceramics might be used as a substrate due to their chemical stability and mechanical strength.
When choosing a substrate for a microfluidic master mold, considerations will generally include the required feature sizes and aspect ratios, the fabrication techniques available, and the intended application of the microfluidic device. It is always a good practice to choose a substrate that is most compatible with the intended downstream applications and the fabrication facility’s capabilities.
What Is Microfluidics?
Microfluidics is a technique used in experiments that require little or no sample to complete. The process uses 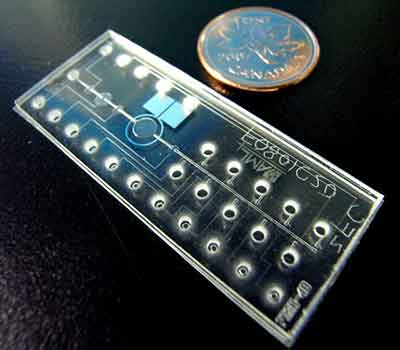 fewer materials and requires less time than larger experiments, so the costs are much lower and the overall precision is higher. Because fewer samples are needed, it improves the accuracy of experiments and decreases the detection limit of a compound. Read on to learn more. But first, let's define what is microfluidics.
fewer materials and requires less time than larger experiments, so the costs are much lower and the overall precision is higher. Because fewer samples are needed, it improves the accuracy of experiments and decreases the detection limit of a compound. Read on to learn more. But first, let's define what is microfluidics.
Materials used in Microfluidics
Thermoplastic polymers (TPE) are highly cross-linked plastics that exhibit high mechanical strength. The material is rigid and has poor elasticity. It is also insoluble in certain solvents and is hydrophobic. TPE valves are often made of this material. The fabrication of TPE valves is similar to that of PDMS valves. These materials are ideal for microfluidic applications because of their excellent mechanical and thermal stability.
One of the most common materials used in microfluidics is PDMS, which is inexpensive and easy to implement. PDMS microstructures are fabricated on molds using photolithography or conventional machining processes. Multiple layers of PDMS are stacked to produce complex microfluidic designs. PDMS is also a great choice for prototyping. It is ideal for a variety of applications, including microfluidics for medical applications.
Laminar Glow
A fundamental principle of microfluidics is laminar flow, which is characterized by a'single' channel containing one or more layers of fluid. This property allows the flow in a microfluidic device to enter from the right, travel through the device and exit in the same direction. The flow rate ratio of the two phases determines the laminarity of the flow.
When two microfluidic channels are connected with a thin wall, fluid flows in a perpendicular regime. In this configuration, the flow rate is controlled by an external force, the aspect ratio of the channels, and by introducing additional fluid. This allows microfluidic devices to achieve high separation rates and to manipulate mixing at the desired points. A microfluidic device is also used to separate individual components of a mixture.
Flows in the microfluidics field are generally characterized by very low Reynolds numbers. The Reynolds number (Re) is the most important dimensionless parameter in forced flows. It is the ratio of the inertial force density to the viscous force density. This parameter helps determine which flow regime is applicable in microfluidics. A microfluidic device typically has a length scale of L0.
Microfluidics Surface Tension
There is a growing body of knowledge on the role of surface tension in microfluidics. Surfactants are widely used in microfluidics as a means of stabilising emulsions, but their role in the generation of micro-droplets and bubbles remains poorly understood. In addition, the increased knowledge on surfactants will spur the development of surface rheology at a nanoscale, which will allow measurements of surface properties with unprecedented resolving power. To that end, this training course will present the fundamentals of the interdisciplinary field of microfluidics and surface rheology.
While microfluidics was previously unknown to the general public, it has recently been revolutionized by the trend of miniaturization. Moore's law says that the number of transistors in a single integrated circuit doubles every two years, so it is no surprise that microfluidics is the logical extension of this trend. With microfluidics, devices requiring a liquid flow can be produced using tiny chips.
What Do Microfluidics Cost
The price of biological reagents, such as angiogenin protein, is one of the main reasons why microfluidics are becoming more popular. For example, PS12/ml for the most expensive wine, while PS120/ml for angiogenin protein. Using microfluidics to shrink experiments and make them more affordable is an obvious solution to this problem. The science of microfluidics uses tiny streams of fluid to create automated processes.
The Microfluidics Prototype Market report provides in-depth information about the competitive landscape. It includes the current market size and forecast, and identifies future market trends. It also covers cost analysis and market share by application and region. It also includes a competitive landscape and key players' profiles. The report helps companies and individuals determine their position in the Microfluidics Prototype market. To gain more insight into the Microfluidics Technology Market, purchase the report now.
What is PDMS for Microfluidics?
What is PDMS for microfluidic devices? Using this material to create a microfluidic device can greatly improve research and development efforts. The material's transparency, coupled with a variety of permeability and biocompatibility properties, make it a great choice for microfluidic applications. It can also be used in a variety of applications for which it can be useful, such as microheaters and sensors.
In order to cast a PDMS component, you need a petri dish and a piece of acrylic. This can be done by making a mold of the original microfluidic circuit. Alternatively, you can use a microfluidic circuit made from ABS or a similar material. In either case, the PDMS component is cast between two layers of acrylic. Once the mold is created, the PDMS component will cure into the shape you've designed.
The process of bonding PDMS to glass or other surface is easy and effective. Henniker HPT benchtop plasma systems are designed for a repeatable plasma treatment process. They avoid the problems associated with manual needle valve gas inlets. They also ensure consistent conditions for a PDMS bonding process. If you want to learn more about the process, click on the knowledge article links below.
PDMS is a highly hydrophobic elastomer that can be modified to improve its hydrophilicity and inhibit non-specific protein adsorption. In this study, researchers aim to investigate the efficacy of these modification materials in a microfluidic cell-based immunofluorescence assay. The microfluidic device needs to be made of modified PDMS, which allows for cell adhesion, which is essential for the immunostaining procedure.
Video: Microfluidic Fabrication
Advantages of Microfluidics
A key difference between microfluidics and conventional methods is the size of the features. Microfluidic devices are designed to handle sub-microlitre volumes, allowing for experiments of high resolution. This allows them to route predictable laminar flows and match perturbations to biological systems. The fabrication techniques used in microfluidics also don't require highly specialized engineering facilities. These benefits have fueled their widespread adoption.
The physical properties of flow vary greatly at different scales, and the behavior of a fluid differs as well. Flow in microfluidic devices is characterized by a low Reynolds number (Re), which measures the ratio of inertial forces versus viscous forces in a fluid. A low Reynolds number indicates laminar flow. Laminar flow has predictable flow patterns and is suitable for microfluidics.
Microfluidic devices have many advantages. They are inexpensive and can be used to analyze nanoliter-sized volumes. They can reduce the size of particle sizes by several orders of magnitude. The resulting nanoliter-scale volumes can be analyzed quickly and efficiently. A common limitation of microfluidic devices is the relatively low processing volumes. For high-volume applications, mesofluidic approaches might be a better option.
Another advantage of microfluidic devices is their ability to control separations and mixing. The device's ability to separate components of a mixture is also beneficial, as it allows laminar flow to continue. This allows researchers to separate cells in a red blood flow using hydrodynamic lift. These devices also help analyze proteins in a variety of systems. A microfluidic chip is one such application.
What is Closed Channel Microfluidics?
Closed channel microfluidics is a technique for liquid manipulation in a microchannel. This method uses
 positive displacement pumps, such as syringe pumps, to drive fluid through the microchannels. Fluid velocity at the walls of the microchannels must be zero to produce a parabolic velocity profile. This flow allows manipulation of the culture system. The advantages of closed channel microfluidics are many, and these technologies have wide-ranging applications in biology, chemistry, and diagnostics.
positive displacement pumps, such as syringe pumps, to drive fluid through the microchannels. Fluid velocity at the walls of the microchannels must be zero to produce a parabolic velocity profile. This flow allows manipulation of the culture system. The advantages of closed channel microfluidics are many, and these technologies have wide-ranging applications in biology, chemistry, and diagnostics.
Research Clients have Used the Following Wafer for Their Research: Under Oil Open-Channel Microfluidics Empowered by Exclusive Liquid Repllency
Si Item #1116 - Buy Online!
100mm P/B (100) 10-20 ohm-cm SSP 500um Prime Grade
Laminar Flow Allows Manipulations with the Culture System
In closed channel microfluidics, laminar flow allows for precise cellular manipulations. The system's patented design provides laminar flow for single cell and subcellular analysis, as well as temporal analysis of signaling and cellular processes. The resulting culture system can be used for a variety of purposes including drug discovery, cell and gene therapy, and pharmacology.
The laminar flow pattern is a key feature of microfluidics. This configuration enables liquids to flow side by side in microchannels. The laminar flow allows precise manipulations with the culture system, as well as the fluids' high-velocity profiles. In microfluidics, a laminar flow regime is achieved by a low Reynolds number.
The cellular samples were initially trapped in a loading spot when the channel was relatively small. In open systems, cells entered the channel following the contour line, but this resulted in a saddle-like geometry at the intersection of the microchannel and the spot. The cellular samples entered the channel along this contour line, which made it possible to manipulate the culture system.
The double ELR provides the greatest virtual barrier between oil, media, and aqueous solutions. Flow rates of four ml/min are comparable to those in vessels of 100 mm. These results further extend the use of open microfluidic systems in biomedical research. They can also be used for research on the invasion of the blood-brain barrier.
The under-oil open-channel microfluidics has many benefits for cellular research. In addition to providing versatile control over the culture environment, this system allows open physical access to the samples. Its unique characterization of dispersion also allows for the study of fungal biofilms, which are difficult to study in closed channel microfluidics. In closed channel microfluidics, fungal biofilms adhere to surfaces and compromise the device's function.
High degree of Fluidic Control
In confined open channels, high fluidic control is possible with the use of novel open structures. This is analogous to digital microelectronics where discrete unit-volume droplets are manipulated on a substrate using electrowetting. Nonetheless, the range of fluidic operations is limited. Active control can be beneficial as it facilitates more complex fluidic handling and allows for smaller spatial footprints.
Closed channels can be constructed with a variety of materials, including silicone rubber, and polydimethylsiloxane. These materials are elastic, and the presence of bubbles can cause surface waves. These waves can propagate along the channel walls, exciting Rayleigh waves. These waves are induced by the pressure of bubbles interacting with their surroundings. The presence of microfluidic channels in biological samples allows them to be used for various research and development applications.
The resulting microfluidic systems can transport, mix, and separate fluids. Passive microfluidics rely on capillary forces to control the flow, whereas active microfluidics involves defined manipulation of working fluid using active components. Besides passive microfluidics, active microfluidics has a number of applications, such as continuous flow supply, dosing, and flow direction.
Electrowetting of dispersions is a powerful method of confined microfluidic systems, but it is not suitable for high-throughput channel packing or multi-flow based actions. Electrowetting of dispersions is an excellent method for confined microfluidics, but its limitations include low fluidic control and a lack of scalability. Open channel microfluidics, on the other hand, allows for high-resolution measurements of pathogenic fungal biofilm.
Self-Cleaning
Self-cleaning closed channel microfluids are devices that clean themselves through a peristaltic process. These devices have a variety of applications, including sensing toxins, DNA sequence analysis, and inkjet printing devices. A dedicated review of these devices is available. This article introduces the new concept and its applications. Read on to learn more. This is a new approach to closed channel microfluidics.
One of the main goals of this research is to develop self-cleaning closed channel microfluidic devices that are effective at controlling the flow rate. The most common configuration of closed channels is a wetting-dewetting dichotomy with three to four hydrophilic walls and one hydrophobic cover. This chemistry results in spontaneous filling of the wider microchannel while leaving the narrower microchannel unfilled.
Several advances in this field have led to the development of capillaric elements and circuits. The advantages of these devices include their minimally-instrumented operation, small sample volumes, and cost. Moreover, they are suited for point-of-care use, which can save time and money. Ultimately, these devices can help to improve global health care by delivering precise and accurate measurements.
Microfluidic Applications in Biology, Diagnostics, Chemistry
In this section, we will discuss some of the most common microfluidics applications in biology, chemistry, and diagnostics. One of these applications is the isolation of cellular samples. Although urine has a low solids content, it contains a variety of particulates. These particles include cells, casts (blood cells that have passed through the renal tubules), proteins, and a variety of crystals. Generally, these particulates have varying sizes, electromagnetic properties, and staining propensities. The study of urinary sediment is a challenging endeavor, but can yield critical diagnostic information.
Another application of open microfluidics is the measurement of dispersion of pathogenic fungal biofilms. These applications require highly accurate measurements of the dispersed cells. For example, it is possible to monitor the dispersion of the biofilm and categorize them based on their size and shape. This microfluidic system can explore the parameter space governing dispersion and how genetic variations and mutations affect it.
Nanostrips are a new reagent developed for closed-channel microfluidics. They are similar to standard urinalysis test strips except they are billions of times smaller and contain multiple sensor locations. Each sensor location responds differently to a target. Embedded reagents are fluorescent dyes, aptamers, and antibodies. Several nanostrips are able to measure thousands of targets from a single sample.
The ability to manipulate a wide range of flow rates and steady flows is a crucial requirement for successful microfluidics. Because open channels have no physical walls, robust confinement of fluids is challenging. Fortunately, the double ELR provides the highest virtual barrier to fluid. Moreover, stable flow is important for biomedical research, mass transport, and mechanical cues.
Biphasic Applications
Microfluidic chips with biphasic applications enable multiple functions to be integrated on the same platform. This approach allows the integration of different processes like cell injection, culture, lysis, separation, and detection. There are many options for the material used in microfluidic chips, but the most popular is polydimethylsiloxane, a high-molecular-weight organic silicon compound. This polymerized silicon compound has several advantages but has many limitations in microfluidics.
A new approach to microfluidics enables the phase transfer of stoichiometric reagents, and it enables biphasic catalytic reactions. Recently, Theberge et al. described a fluorous microfluidic system for the Suzuki cross-coupling reaction. They used an amphiphilic ligand with a fluorinated tail and a hydrophilic guanidine head as catalysts. They obtained the Suzuki cross-coupling products in excellent yields. The fluorous phase could be recycled in a closed loop system, with droplets pooling at the end of the reaction channel to recycle the catalytically active fluorous phase.
This microfluidic system allows precise spatiotemporal control of the cellular microenvironment, allowing researchers to create physiologically relevant environments for signals. By scaling liquids and generating a gradient of concentration, these chips can replicate the physiological length scale and mechanical force of fluid flow. These capabilities have attracted the attention of the pharmaceutical industry, regulatory agencies, and national defense agencies. It is also possible to manipulate various parameters in the cellular microenvironment, allowing for precise quantitative analysis of how it affects cells.
Another type of microfluidic technology is suspended microfluidics. These devices can create multiplexed systems for biochemical assays and can utilize many fluids and open geometries. A biphasic microfluidic device can also be designed for a wide range of fluids and temperatures. Once the device is ready for use, a variety of applications await.
Video: How to Make a Microfluidic Device
What are Advantages of Open Channel Microfluidics?
The advantages of open channel microfluidics are numerous. Its high flow rates, low operating voltage requirement, ability to break surface tension, and applications are some of the key advantages. Read on to learn more about this fascinating technology. Listed below are the most important features of open channel microfluidics. Weigh the advantages of open channel microfluidics compared to other microfluidics techniques.
High Flow Rates
Microfluidic systems based on open channel design should incorporate valves that allow for control of the fluidic properties of the channel. The current study demonstrates a static fluidic condition for a 100-mm feature. The high flow rates achieved with open channels are similar to those of closed microfluidic systems, but the two types differ fundamentally in terms of fluidic control. Open microfluidics are a promising approach to high-throughput cellular samples.
To study the effect of pressure drop on the flow rate in an open channel microfluidic system, we set up a microchannel with a single syringe pump and measured the maximum volumetric flow rate. Poiseuille's law defines the volumetric flow rate as the maximum Laplace pressure difference between the inlet and outlet points. It was also observed that the critical flow rate was influenced by the length of the open channel.
The advantages of open channel microfluidics include its low cost, simplicity of design, and ease of use. With no bonding required to form the channel, these devices are inexpensive and easy to manufacture. In addition, open microfluidic devices also eliminate the risk of bubble trapping and associated device failures. These features reduce the barrier for end users to adopt the technology. These advantages are the key to open channel microfluidics' success.
Double ELR offers a potential solution to these challenges. It enables channel filling at smaller scales than previously reported, thus mitigating the loss due to evaporation. Open microfluidic devices have traditionally been limited by volume loss due to evaporation, and this study's micrometer-scale characterization does not approach theoretical limits. It also represents the limit of the existing patterning techniques available on the market.
Low Operating Voltage Requirement
Open microfluidic devices do not require any bonding to another surface and are thus very easy to construct and operate. Moreover, the low operating voltage requirement of these devices significantly reduces the barriers to adoption for end users. A low operating voltage is also very important in healthcare applications. However, this disadvantage can be overcome by using LDEP techniques to reduce the operating voltage requirement. By applying an LDEP technique, Oh and his team have successfully addressed these challenges.
The double ELR process overcomes these technical challenges by enabling channel filling on a smaller scale than previously reported, and it mitigates volume loss due to evaporation, which has severely limited the functionality of open channel microfluidic devices. While the micrometer scale achieved by this study does not approach theoretical limits, it is the limit of readily available patterning techniques. Hence, enabling technologies are needed to overcome these barriers.
Another technique for lowering the operating voltage requirement of open channel microfluidics is based on a liquid bridge. The process enables adding an initial volume to a valve to bridge the upstream and downstream channels. In a similar manner, active pumping is also possible to maintain steady flow across the microchannel. However, this method is more complex and requires careful consideration of connection dynamics.
Another way to reduce the operating voltage of open channel microfluidics is by using an open system with a low h/w ratio. Under this setup, cellular samples are effectively contained within the microchannel. The cellular sample is suspended in the liquid and is surrounded by an oily membrane, which prevents bacterial growth. This feature makes it possible to study the synchronized behavior of different colonies with minimal disruption.
Ability to Break Surface Tension
Microfluidic devices incorporating under oil have several unique features. These include an open physical access and microfilter function, lossless on-chip sample processing, and a low adoption barrier. In addition, the under oil device is particularly useful for studying fungal biofilms, which are notoriously difficult to study in closed-channel microfluidic devices due to their tendency to adhere to the surface.
In open systems, the channel height is gradually reduced, resulting in a saddle-shaped geometry at the connection of the microchannel to the spots. This saddle-shaped geometry facilitates the entry of cells and other fluids, even those that exhibit weak surface tension. It also eliminates the risk of device failure due to bubble formation. In addition, open devices enable fluid access from any point in the channel, allowing them to be easily added or removed.
Open channel microfluidics is rapidly advancing, with a wide range of applications in laboratories around the world. The open microfluidic capillary system is a versatile and highly efficient tool for fluid manipulation. It is easy to fabricate and reliable to operate. It is widely used in a wide variety of applications, including biomedical research and diagnostics. This type of system is particularly useful in resource-constrained settings.
A photo mask was designed in Adobe Illustrator and sent to Fineline Imaging to create a master with microchannel features. A 4'' silicon wafer (ID 1116) was cleaned in acetone, rinsed with isopropyl, and dried with nitrogen before use. The silicon wafer was then baked on an EchoTherm HP30 hotplate at 95dec for 30 min.
What are Some Open Channel Microfluidic Applications?
An open channel microfluidic device consists of a set of channels that are bonded together with an organic carrier phase. As the name implies, the fluidic devices are open, allowing direct access to the sample. The open design enables a streamlined process for large-scale screening experiments. Open microfluidic devices are a great way to eliminate the hassles and costs associated with manual processing, which can negatively impact assay time and sample loss.
The general platform design of the open channel system is used to translate aqueous droplets via capillary flow or an organic carrier phase. Typical examples of open microfluidics include aqueous droplets in blue and organic carrier fluid in yellow. A cross-sectional schematic of the open channel microfluidics system can be found in Figure 1.
Another type of open channel microfluidics is known as "under oil open-channel" microfluidics. This device combines miniaturization and reversible valving to achieve high-throughput channel packing and micron-scale fluid manipulation. Unlike the enclosed channel microfluidics system, open-channel systems can accommodate a wide range of liquids and allow for multi-flow-based actions, such as drug screening.
The advantages of open channel microfluidics over closed-channel systems include direct sample access and reduced manufacturing costs. Furthermore, open microfluidic capillary systems enable use of thermoplastic materials and low-cost disposables, making them a good option for diagnostics in under-resourced settings. It also enables the use of open channel microfluidic systems for biomedical applications. The potential for such applications is endless.
Open microfluidics are an emerging technology that allows the integration of pipetting systems, robotics, and advanced valving methods. The open microfluidics technology has many exciting future research directions. New publications on the field have just been published, so the information presented here is not exhaustive. Nonetheless, open microfluidics is rapidly gaining ground in the research community. These developments promise to bring many of the advantages of closed-channel droplet-based microfluidics to open systems.
Challenges
Traditionally, open channel systems are passively operated, which means that the fluidic operations must be predefined during fabrication. This limitation limits the scope of fluid processing and results in large spatial footprints. Active control, on the other hand, enables complex fluidic handling while promoting smaller footprints. OMEF technology combines confined open channels with active liquid actuation. This method has several advantages over conventional closed channel systems.
For example, the ability to control the flow rate of a fluid in an open channel microfluidic system is critical for the accurate analysis of cellular samples. In addition to allowing precise manipulation of cellular samples, extreme wettability enables advanced mass transport in open channels. The versatility of open channel microfluidic systems offers many easy-to-explore applications, including biofilm dynamics and host-microbe interactions.
With its open access to the entire channel, nanotubes and other materials are easier to handle than traditional silicon-based microfluidics. For instance, using open channels in biosensing applications would allow researchers to screen for compounds that inhibit virulence and dispersive capacity of bacteria and fungi. Moreover, open channel microfluidics can allow researchers to study fungal biofilms, which can cause a compromise in the device's functionality.
Achieving the desired steady flow rate of a fluid in an open channel microfluidic device is extremely challenging. Current open channel microfluidics technologies have limited scale, making it difficult to obtain low-flow and continuous flow in open microfluidics. Nevertheless, recent advances in surface patterning techniques are making the possibility of sub-micrometer-scale microfluidics a reality.
Video: Open Channel Microfluidics
Keywords:
- microfluidic channels
- microfluidic photoablation
- microfluidic applications
- microfluidic network
- capillary flow
- capillary systems
- tube fabrication
- capillary pressure
- microfluidic device
- open microchannel
- trapped bubbles
- channel patterns
- pcb wafers
- vessel outlet
- hydrostatic pressure
