Is Indium Phosphide Cubic Crystal?
A Chemical Engineering researcher working on molecular beam epitaxy of thin films for exploring electronic, optical, and magnetic properties of low dimensional defects, including growth of II-VI and magnetic oxide semiconductors requested help with the following questions and needed a quote.
I am interested in your InP wafers with item #K661.
Question:
- What does the A in [111A] stand for?
- Is InP Is this a cubic crystal wafer?
Answer:
- In face is polished and P face is etched and adequately for [111B] P face is polished and In is face etched
- Yes, it is cubic crystal wafer
Reference #268498 for specs and quantity.
Get Your Quote FAST! Or, Buy Online and Start Researching Today!
K661 wafers are sold out. We offer HQ20 now:
Item Qty. Description
HQ20. 5/10/30 Indium Phosphide wafers, P/E 2"Ø×350±25µm,
Undoped InP [111B]±0.5°,
Nc<3E16/cc, Bow<30µm,Warp<30µm.
One-side-polished, back-side matte etched, EJ Flats, Surface roughness<15A.
Sealed under nitrogen in single wafer cassette.
What Are Cubic Crystals?
In the context of semiconductors, cubic crystals refer to materials with a crystal structure where the atoms are 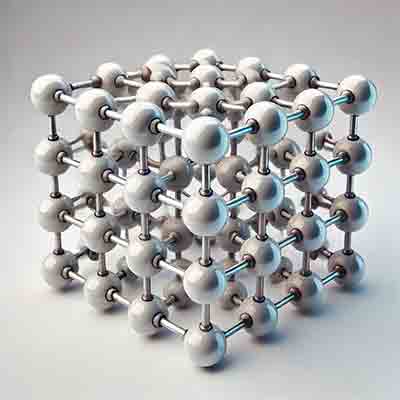 arranged in a cubic lattice. This type of lattice is one of the seven basic crystal systems in crystallography and is characterized by having three equal axes that intersect at right angles (90°). There are three main types of cubic crystal structures:
arranged in a cubic lattice. This type of lattice is one of the seven basic crystal systems in crystallography and is characterized by having three equal axes that intersect at right angles (90°). There are three main types of cubic crystal structures:
1. Simple Cubic (SC)
- Atoms are located at each corner of the cube.
- It is the simplest arrangement but not common in semiconductors due to its low packing density.
- Example: Polonium (not a typical semiconductor).
2. Body-Centered Cubic (BCC)
- In addition to atoms at each corner, there is one atom at the center of the cube.
- Higher packing density than simple cubic.
- Example: Not common in semiconductors but found in metals like iron (in some phases).
3. Face-Centered Cubic (FCC)
- Atoms are located at each corner and at the center of each face of the cube.
- Higher packing density compared to SC and BCC.
- Example: Not directly applicable to semiconductors in this form.
Special Case for Semiconductors: Diamond Cubic
- A derivative of the FCC structure.
- Each atom in the FCC lattice is covalently bonded to four other atoms in a tetrahedral configuration.
- Common in elemental semiconductors like silicon (Si) and germanium (Ge).
- High symmetry and excellent electronic properties make this structure ideal for semiconductor applications.
Examples of Cubic Crystals in Semiconductors
- Silicon (Si): Diamond cubic.
- Germanium (Ge): Diamond cubic.
- Gallium Arsenide (GaAs): Zinc blende (similar to diamond cubic but with two types of atoms, Ga and As).
- Gallium Nitride (GaN): Can also crystallize in cubic zinc blende (though hexagonal wurtzite is more common).
Cubic structures are essential in semiconductors due to their symmetry, which contributes to isotropic electronic properties and simplifies fabrication processes. The high symmetry also affects electron mobility and band structure, which are critical for the performance of semiconductor devices.
Indium Phosphide (InP) is a III-V compound semiconductor that crystallizes in the zinc blende (cubic) crystal structure. This structure is similar to the diamond cubic lattice, but it consists of two types of atoms (indium and phosphorus) instead of a single type.
Key Features of InP Crystal Structure
-
Zinc Blende Structure:
- Indium (In) and phosphorus (P) atoms alternate in a face-centered cubic (FCC) lattice.
- Each atom is tetrahedrally coordinated, meaning each In atom is covalently bonded to four P atoms, and vice versa.
-
Lattice Constant:
- The lattice constant for InP is approximately 5.8687 Å.
-
Symmetry:
- High cubic symmetry contributes to isotropic properties in the absence of external stress.
-
Electronic Properties:
- Direct bandgap: InP has a direct bandgap of about 1.34 eV at room temperature, making it highly efficient for optoelectronic applications like lasers and photodetectors.
Applications of InP
Indium phosphide is widely used in high-frequency and optoelectronic applications due to its excellent electronic and optical properties. Key applications include:
- Optoelectronics:
- Used in lasers, photodiodes, and LEDs for fiber-optic communication systems.
- High-Speed Electronics:
- Employed in high-frequency transistors, such as high-electron-mobility transistors (HEMTs) and heterojunction bipolar transistors (HBTs).
- Solar Cells:
- Used in multijunction solar cells for space applications due to its high efficiency and radiation hardness.
Comparison with Other III-V Semiconductors
- Like gallium arsenide (GaAs), InP has a zinc blende structure, but its smaller bandgap and different lattice constant make it more suitable for longer-wavelength applications.
- InP's lattice constant is also better matched with certain other III-V materials (e.g., InGaAs), enabling the growth of high-quality heterostructures.
InP's cubic crystal structure is a fundamental reason for its success in semiconductor technology, providing the basis for its remarkable electrical and optical performance.
 arranged in a cubic lattice. This type of lattice is one of the seven basic crystal systems in crystallography and is characterized by having three equal axes that intersect at right angles (90°). There are three main types of cubic crystal structures:
arranged in a cubic lattice. This type of lattice is one of the seven basic crystal systems in crystallography and is characterized by having three equal axes that intersect at right angles (90°). There are three main types of cubic crystal structures: